Deck 15: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/85
Play
Full screen (f)
Deck 15: Nuclear Chemistry
1
According to Albert Einstein's theory of relativity, energy (E) and mass (m) are related by the following equation: E = mc
where c is the speed of light, or 3.00 × 108 m/s.
where c is the speed of light, or 3.00 × 108 m/s.
False
2
Dubnium has an atomic number of 105. When dubnium-262 emits an alpha particle, _____ is the resulting daughter isotope.
A)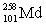
B)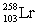
C)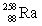
D)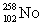
E)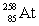
A)
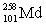
B)
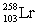
C)
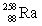
D)
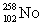
E)
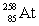
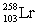
3
Neptunium has an atomic number of 93. When _____ emits an alpha particle, neptunium-239 is the resulting daughter isotope.
A)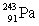
B)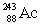
C)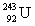
D)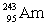
E)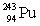
A)
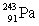
B)
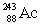
C)
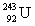
D)
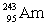
E)
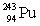
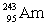
4
Nuclear fusion is the breaking down of large nuclei into smaller nuclei, usually with the release of excess neutrons.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
5
An 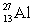 atom has13 neutrons in its nucleus.
atom has13 neutrons in its nucleus.
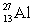 atom has13 neutrons in its nucleus.
atom has13 neutrons in its nucleus.
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
6
Only radioactive isotopes have a half-life.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
7
Uranium-238 can absorb a neutron and undergo a fission reaction to produce an atom of cesium-135 and an atom of rubidium-96. The balanced nuclear equation for the process is 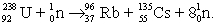

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
8
A 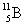 atom has 5 protons in its nucleus.
atom has 5 protons in its nucleus.
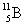 atom has 5 protons in its nucleus.
atom has 5 protons in its nucleus.
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
9
An alpha particle is a collection of four protons and five neutrons and is equivalent to a beryllium nucleus.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
10
The product in the nuclear equation is the daughter isotope.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
11
Polonium has an atomic number of 84. When polonium-209 emits an alpha particle, the resulting daughter isotope is _____.
A)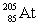
B)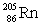
C)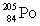
D)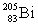
E)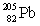
A)
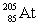
B)
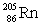
C)
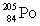
D)
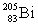
E)
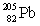

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
12
An isotope with a longer half-life is generally considered more radioactive.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
13
The reactant in the nuclear equation is the parent isotope.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
14
Francium has an atomic number of 87. Which of the following is the nuclear equation that represents the radioactive decay of francium-223 by alpha particle?
A)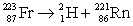
B)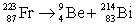
C)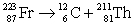
D)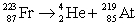
E)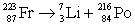
A)
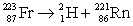
B)
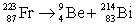
C)
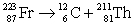
D)
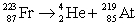
E)
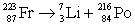

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
15
A beta particle is a neutron emitted from the nucleus.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
16
Gamma rays are high-energy electromagnetic radiation given off in radioactive decay.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
17
Complete the reaction. 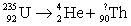
A) 230
B) 231
C) 232
D) 233
E) 234
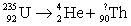
A) 230
B) 231
C) 232
D) 233
E) 234

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
18
Actinium has an atomic number of 89. When _____ emits an alpha particle, actinium-227 is the resulting daughter isotope.
A)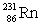
B)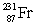
C)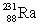
D)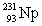
E)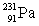
A)
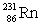
B)
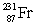
C)
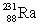
D)
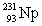
E)
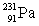

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
19
Alpha particles penetrate more than beta particles.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
20
A chain reaction is a process that generates more reaction pathways for each previous reaction.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
21
A sample of curium has an activity of 2,450 Bq. If the half-life of curium is 24.0 s, how long before its activity is 25.0 Bq?
A) 591 s
B) 195 s
C) 159 s
D) 951 s
E) 915 s
A) 591 s
B) 195 s
C) 159 s
D) 951 s
E) 915 s

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
22
The half-life of a radioactive sample is 11.0 s. If the sample initially contains 25.0 g of the radioactive sample, how much remains after 54.0 s?
A) 0.00338 g
B) 0.0338 g
C) 0.833 g
D) 0.383 g
E) 0.338 g
A) 0.00338 g
B) 0.0338 g
C) 0.833 g
D) 0.383 g
E) 0.338 g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
23
Radium has an atomic number of 88. Radium-226 is the daughter isotope formed when _____ emits a beta particle.
A)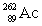
B)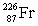
C)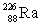
D)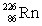
E)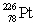
A)
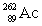
B)
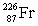
C)
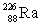
D)
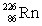
E)
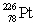

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
24
A sample of radon has an activity of 90,000 Bq. If the half-life of radon is 15 h, how long before the sample's activity is 5,625 Bq?
A) 60 h
B) 90 h
C) 15 h
D) 30 h
E) 45 h
A) 60 h
B) 90 h
C) 15 h
D) 30 h
E) 45 h

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
25
Radium has an atomic number of 88. _____is the daughter isotope formed when radium-226 emits a beta particle.
A)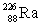
B)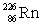
C)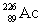
D)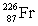
E)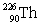
A)
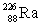
B)
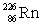
C)
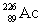
D)
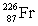
E)
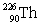

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
26
Lawrencium has an atomic number of 103. _____is the daughter isotope formed when lawrencium-262 emits a beta particle.
A)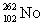
B)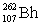
C)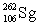
D)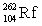
E)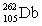
A)
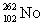
B)
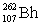
C)
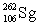
D)
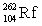
E)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
27
A sample of radon gas has an activity of 140.0 mCi. If the half-life of radon is 1,500.0 y, how long before the activity of the sample is 10.0 mCi?
A) 1,750 y
B) 1,510 y
C) 7,510 y
D) 5,170 y
E) 5,710 y
A) 1,750 y
B) 1,510 y
C) 7,510 y
D) 5,170 y
E) 5,710 y

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
28
If the half-life of tritium (hydrogen-3) is 12.3 y, how much of a 0.0666 g sample of tritium is present after 50.0 y?
A) 8.93*10-3 g
B) 9.83 * 10-3 g
C) 3.98 * 10-3 g
D) 8.39 * 10-3 g
E) 8.93 *103 g
A) 8.93*10-3 g
B) 9.83 * 10-3 g
C) 3.98 * 10-3 g
D) 8.39 * 10-3 g
E) 8.93 *103 g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
29
How long does it take for 5.05 g of a radioactive isotope to decay to 0.0505 g if its half-life is 20,000 y?
A) 205,000 y
B) 505,000 y
C) 313,000 y
D) 331,000 y
E) 133,000 y
A) 205,000 y
B) 505,000 y
C) 313,000 y
D) 331,000 y
E) 133,000 y

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
30
The half-life of a radioactive sample is 60.0 y. If the sample weighs 0.893 g initially, how much remains after 420.0 y?
A) 0.00698 g
B) 0.00968 g
C) 0.00986 g
D) 0.00689 g
E) 0.00896 g
A) 0.00698 g
B) 0.00968 g
C) 0.00986 g
D) 0.00689 g
E) 0.00896 g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
31
Energies of gamma rays are typically expressed in units of megaelectron volts (MeV), where 1 MeV = 1.602 × 10−13 J. Energies of gamma rays emitted when nitrogen-11 gives off a beta particle is 1.508 × 10-12 J. What is its energy in MeV?
A) 9.14 MeV
B) 4.19 MeV
C) 9.41 MeV
D) 1.49 MeV
E) 4.91 MeV
A) 9.14 MeV
B) 4.19 MeV
C) 9.41 MeV
D) 1.49 MeV
E) 4.91 MeV

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
32
Complete the following reaction. 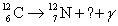
A)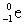
B)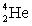
C)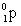
D)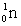
E)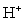
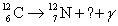
A)
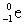
B)
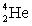
C)
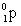
D)
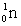
E)
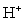

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
33
Uranium has an atomic number of 92. Uranium-238 is the daughter isotope formed when _____ emits a beta particle.
A)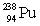
B)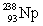
C)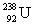
D)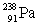
E)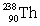
A)
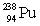
B)
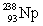
C)
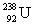
D)
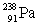
E)
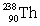

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
34
_____ = 3.7 * 1010 decays/s
A) 1 rad
B) 1 rem
C) 1 Ci
D) 1 cal
E) 1 coulomb
A) 1 rad
B) 1 rem
C) 1 Ci
D) 1 cal
E) 1 coulomb

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
35
A sample of radon has an activity of 80,000 Bq. If the half-life of radon is 15 h, how long before the sample's activity is 5,000 Bq?
A) 10 h
B) 20 h
C) 45 h
D) 30 h
E) 60 h
A) 10 h
B) 20 h
C) 45 h
D) 30 h
E) 60 h

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
36
Energies of gamma rays are typically expressed in units of megaelectron volts (MeV), where 1 MeV = 1.602 × 10−13 J. Energies of gamma rays emitted when oxygen-13 gives off a beta particle is 0.168 MeV. What is its energy in joules?
A) 6.29 × 10-12J
B) 5.01 × 1012J
C) 1.05 × 10-12J
D) 0.51 × 1014 J
E) 2.69 × 10 -14 J
A) 6.29 × 10-12J
B) 5.01 × 1012J
C) 1.05 × 10-12J
D) 0.51 × 1014 J
E) 2.69 × 10 -14 J

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
37
The half-life of carbon-11 is 20.3 min. If 5.00 g of carbon-11 is left in the sample after 59.3 min, what mass of carbon-11 was present initially?
A) 93.7 g
B) 79.3 g
C) 73.9 g
D) 37.9 g
E) 39.7 g
A) 93.7 g
B) 79.3 g
C) 73.9 g
D) 37.9 g
E) 39.7 g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
38
A sample containing carbon-14 contains 5.30 × 10−6 g of carbon-14 in it. If the age of the sample is 15,800 y, how much carbon-14 did it have originally? The half-life of carbon-14 is 5,730 y.
A) 3.58 * 10-6 g
B) 3.58 * 10-5 g
C) 3.58 * 10-4 g
D) 8.53 *10-5 g
E) 8.53 * 10-4 g
A) 3.58 * 10-6 g
B) 3.58 * 10-5 g
C) 3.58 * 10-4 g
D) 8.53 *10-5 g
E) 8.53 * 10-4 g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
39
How long does it take for 10.0 g of a radioactive isotope to decay to 1.25 g if its half-life is 17.0 d?
A) 15.0 d
B) 51.0 d
C) 17.0 d
D) 71.0 d
E) 30.0 d
A) 15.0 d
B) 51.0 d
C) 17.0 d
D) 71.0 d
E) 30.0 d

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
40
The half-life of americium-241 is 432 y. If 2.00 g of americium-241 is present in a sample, what mass of americium-241 is present after 1,000.0 y?
A) 0.0402 g
B) 0.0204 g
C) 0.204 g
D) 0.402 g
E) 0.420 g
A) 0.0402 g
B) 0.0204 g
C) 0.204 g
D) 0.402 g
E) 0.420 g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
41
Complete the following reaction. 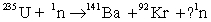
A) 0
B) 1
C) 4
D) 2
E) 3
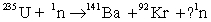
A) 0
B) 1
C) 4
D) 2
E) 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
42
After chemical analysis, a radioactive sample is found to contain 2 g of uranium to every 5 g of thorium, its daughter isotope. If the half-life of uranium is 68.9 y, approximately how old is the sample?
A) 70.0 y
B) 83.7 y
C) 111 y
D) 165 y
E) 125 y
A) 70.0 y
B) 83.7 y
C) 111 y
D) 165 y
E) 125 y

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
43
What is the energy change of this fission reaction? Masses in grams are provided. 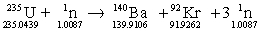
A) 1.087 * 1014 J
B) 1.043 * 1014 J
C) 1.910 * 1014 J
D) 1.071 F* 1014 J
E) 1.926 * 1014 J
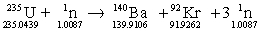
A) 1.087 * 1014 J
B) 1.043 * 1014 J
C) 1.910 * 1014 J
D) 1.071 F* 1014 J
E) 1.926 * 1014 J

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
44
Determine the change in mass for the reaction below. Masses in grams are provided. 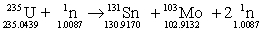
A) -0.2050 g
B) -0.0439 g
C) -0.9170 g
D) -0.9132 g
E) -0.0087 g
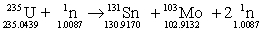
A) -0.2050 g
B) -0.0439 g
C) -0.9170 g
D) -0.9132 g
E) -0.0087 g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the energy change for the reaction below. Masses in grams are provided. 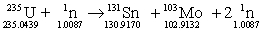
A) 1.044 * 1013 J
B) 1.845 * 1013 J
C) 1.009 * 1013 J
D) 1.917 * 1013 J
E) 1.005 * 1013 J
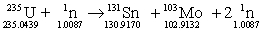
A) 1.044 * 1013 J
B) 1.845 * 1013 J
C) 1.009 * 1013 J
D) 1.917 * 1013 J
E) 1.005 * 1013 J

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
46
Describe beta particle emission.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
47
Describe alpha particle emission.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
48
After chemical analysis, a radioactive sample is found to contain 1.00 g of francium to every 2.50 g of astatine, its daughter isotope. If the half-life of francium is 4.18 min, approximately how old is the sample?
A) 7.65 min
B) 7.56 min
C) 5.67 min
D) 5.76 min
E) 6.57 min
A) 7.65 min
B) 7.56 min
C) 5.67 min
D) 5.76 min
E) 6.57 min

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
49
A radioactive sample has a half-life of 28.1 y. If 98.0 Bq of the sample were allowed to decay for 27.0 y, what would the activity of the remaining sample be?
A) 53.7 Bq
B) 537 Bq
C) 0.735 Bq
D) 7.35 Bq
E) 50.4 Bq
A) 53.7 Bq
B) 537 Bq
C) 0.735 Bq
D) 7.35 Bq
E) 50.4 Bq

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
50
If a radioactive sample has an activity of 65.0 nCi, how many disintegrations per second are occurring?
A) 1,240 disintegrations/s
B) 2,140 disintegrations/s
C) 2,410 disintegrations/s
D) 1,242 disintegrations/s
E) 1,420 disintegrations/s
A) 1,240 disintegrations/s
B) 2,140 disintegrations/s
C) 2,410 disintegrations/s
D) 1,242 disintegrations/s
E) 1,420 disintegrations/s

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the change in mass for the reaction below. Masses in grams are provided. 
A) -0.0087 g
B) -1.0087 g
C) -1.1897 g
D) -0.0439 g
E) -0.9262 g

A) -0.0087 g
B) -1.0087 g
C) -1.1897 g
D) -0.0439 g
E) -0.9262 g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
52
Complete the following reaction. 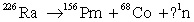
A) 0
B) 1
C) 4
D) 2
E) 3
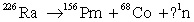
A) 0
B) 1
C) 4
D) 2
E) 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
53
A typical dose of a radioactive sample is 27.0 mCi. How long does it take for the activity to reduce to 0.100 mCi? The half-life of the sample is 211,000 y.
A) 0.17 * 105 y
B) 0.17 * 106 y
C) 1.70 * 105 y
D) 1.70 *104 y
E) 1.70 *106 y
A) 0.17 * 105 y
B) 0.17 * 106 y
C) 1.70 * 105 y
D) 1.70 *104 y
E) 1.70 *106 y

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
54
Define radioactivity.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
55
How long does it take 200.0 mCi of a radioactive sample to decay to 20.0 mCi if its half-life is 11.0 s?
A) 25.4 s
B) 5.36 s
C) 36.5 s
D) 53.6 s
E) 73.0 s
A) 25.4 s
B) 5.36 s
C) 36.5 s
D) 53.6 s
E) 73.0 s

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
56
Complete the following reaction. 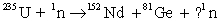
A) 0
B) 1
C) 4
D) 2
E) 3
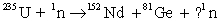
A) 0
B) 1
C) 4
D) 2
E) 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
57
For every mole of radioactive sample that decays, 0.2002 g of mass is lost. How much energy is given off per mole of radioactive sample reacted?
A) 1.028* 1013 J
B) 1.208 * 1013 J
C) 1.802 * 1013 J
D) 2.810 * 1013 J
E) 2.180 *1013 J
A) 1.028* 1013 J
B) 1.208 * 1013 J
C) 1.802 * 1013 J
D) 2.810 * 1013 J
E) 2.180 *1013 J

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
58
For every mole of a radioactive sample that decays, 0.1560 g of mass is lost. How much energy is given off per mole of radioactive sample reacted?
A) 1.04 * 1013 J
B) 4.01 * 1013 J
C) 4.10 *1013 J
D) 0.14 *1013 J
E) 1.40 * 1013 J
A) 1.04 * 1013 J
B) 4.01 * 1013 J
C) 4.10 *1013 J
D) 0.14 *1013 J
E) 1.40 * 1013 J

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
59
A sample of fluorine-20 has an activity of 5.94 mCi. If its half-life is 11.0 s, what is its activity after 55.0 s?
A) 0.816 mCi
B) 0.861 mCi
C) 0.168 mCi
D) 0.186 mCi
E) 0.618 mCi
A) 0.816 mCi
B) 0.861 mCi
C) 0.168 mCi
D) 0.186 mCi
E) 0.618 mCi

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
60
If a radioactive sample has an activity of 8.33 Bq, how many disintegrations per second are occurring?
A) 8.33 disintegrations/s
B) 4.16 disintegrations/s
C) 3.38 disintegrations/s
D) 30.8 disintegrations/s
E) 33.8 disintegrations/s
A) 8.33 disintegrations/s
B) 4.16 disintegrations/s
C) 3.38 disintegrations/s
D) 30.8 disintegrations/s
E) 33.8 disintegrations/s

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
61
Give the various expressions used to determine the final amount remaining in a radioactive sample.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
62
_____ = 3.7 * 1010 decays/s

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
63
Who is the curie named after? Who is the becquerel named after?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
64
Three forms of radioactive emissions are ___________ particle, _____________
particle and ________________ rays.
particle and ________________ rays.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
65
A(n) _____ is a chemical equation that emphasizes changes in atomic nuclei.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
66
Define nuclear fission.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
67
________________ discovered natural radioactivity from uranium ores.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
68
rem = _____ * factor

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
69
Define 1 rad.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
70
________________ can penetrate deeply into matter and can impart large amounts
of energy into surrounding matter.
of energy into surrounding matter.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
71
Explain how a Geiger counter works to detect radiation.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
72
Define 1 becquerel (Bq).

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
73
Define 1 rem.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
74
The breaking apart of an atomic nucleus into smaller nuclei is called _____.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
75
Describe gamma ray emission.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
76
Define 1 curie (Ci).

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
77
Which is more radioactive-an isotope with a long half-life or an isotope with a short half-life?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
78
Explain why the amount left in a radioactive sample after 1,000.0 y is not one-tenth of the amount present after 100.0 y, despite the fact that the amount of time elapsed is 10 times as long.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
79
_____= 100 rad

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
80
Plutonium-239 emits alpha particles and is hazardous when inhaled or ingested. What new element is formed by this alpha emission?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck



