Deck 14: Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/165
Play
Full screen (f)
Deck 14: Organic Chemistry
1
In organic molecules, a bromine atom will normally form how many bonds?
A) 2
B) 3
C) 4
D) 1
E) 0
A) 2
B) 3
C) 4
D) 1
E) 0
D
2
Hydrocarbons containing only single bonds are
A) alkanes.
B) alkenes.
C) aromatics.
D) alkynes.
E) ketones.
A) alkanes.
B) alkenes.
C) aromatics.
D) alkynes.
E) ketones.
A
3
In organic molecules, a sulfur atom will normally form how many bonds?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4
C
4
Which of the following atoms normally has one covalent bond to it?
A) Br
B) O
C) C
D) P
E) Ne
A) Br
B) O
C) C
D) P
E) Ne

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is a methyl group?
A) CH3CH2-
B) CH3-
C) CH2=CH-
D) CH2CH3-
E) CH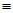 CH-
CH-
A) CH3CH2-
B) CH3-
C) CH2=CH-
D) CH2CH3-
E) CH
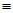 CH-
CH-
Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following atoms normally has one covalent bond to it?
A) At
B) Se
C) Si
D) P
E) Ne
A) At
B) Se
C) Si
D) P
E) Ne

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following atoms normally has one covalent bond to it?
A) Cl
B) Te
C) Si
D) N
E) Ne
A) Cl
B) Te
C) Si
D) N
E) Ne

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
8
Hydrocarbons containing carbon-carbon triple bonds are
A) alkenes.
B) cycloalkanes.
C) alkynes.
D) alkanes.
E) ketones.
A) alkenes.
B) cycloalkanes.
C) alkynes.
D) alkanes.
E) ketones.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
9
In organic molecules, a chlorine atom will normally form how many bonds?
A) 2
B) 3
C) 4
D) 1
E) 0
A) 2
B) 3
C) 4
D) 1
E) 0

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
10
In organic molecules, a carbon atom will normally form how many bonds?
A) 1
B) 4
C) 3
D) 2
E) 0
A) 1
B) 4
C) 3
D) 2
E) 0

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following atoms normally has one covalent bond to it?
A) I
B) O
C) C
D) P
E) Kr
A) I
B) O
C) C
D) P
E) Kr

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
12
When a carbon atom forms four single bonds, the bonds are at what angle to one another?
A) 109.5°
B) 90°
C) 120°
D) 23.5°
E) 60°
A) 109.5°
B) 90°
C) 120°
D) 23.5°
E) 60°

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is an ethyl group?
A) CH3CH2-
B) CH2=CH-
C) CH2CH3-
D) CH3-
E) CH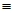 CH-
CH-
A) CH3CH2-
B) CH2=CH-
C) CH2CH3-
D) CH3-
E) CH
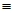 CH-
CH-
Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
14
The study of compounds that contain carbon is called
A) biochemistry.
B) inorganic chemistry.
C) polymer chemistry.
D) organic chemistry.
E) physics.
A) biochemistry.
B) inorganic chemistry.
C) polymer chemistry.
D) organic chemistry.
E) physics.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
15
Organic chemistry studies chemical compounds that contain at least one atom of
A) nitrogen.
B) oxygen.
C) carbon.
D) hydrogen.
E) silicon.
A) nitrogen.
B) oxygen.
C) carbon.
D) hydrogen.
E) silicon.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
16
Hydrocarbons containing all single bonds and a ring in their structural formulas are
A) alkenes.
B) cycloalkanes.
C) alkynes.
D) alkanes.
E) ketones.
A) alkenes.
B) cycloalkanes.
C) alkynes.
D) alkanes.
E) ketones.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
17
In organic molecules, an oxygen atom will normally form how many bonds?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
18
Hydrocarbons containing carbon-carbon double bonds are
A) alkenes.
B) cycloalkanes.
C) alkynes.
D) alkanes.
E) ketones.
A) alkenes.
B) cycloalkanes.
C) alkynes.
D) alkanes.
E) ketones.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
19
C3H4 is an example of a(n)
A) aromatic.
B) alkene.
C) alkyne.
D) alkane.
E) cycloalkane
A) aromatic.
B) alkene.
C) alkyne.
D) alkane.
E) cycloalkane

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
20
In organic molecules, a fluorine atom will normally form how many bonds?
A) 2
B) 3
C) 4
D) 1
E) 0
A) 2
B) 3
C) 4
D) 1
E) 0

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
21
4-Fluoroheptane and 5-fluoroheptane are
A) isomers.
B) the same compound.
C) neither isomers nor the same compound.
A) isomers.
B) the same compound.
C) neither isomers nor the same compound.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
22
Which organic compound is represented by a pentagon?
A) Benzene
B) Cyclopentane
C) Cyclopropane
D) Cyclobutane
E) Methane
A) Benzene
B) Cyclopentane
C) Cyclopropane
D) Cyclobutane
E) Methane

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
23
How many constitutional isomers that are dibromopropanes exist?
A) 5
B) 4
C) 7
D) 3
E) 6
A) 5
B) 4
C) 7
D) 3
E) 6

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following could be the molecular formula for an alkane?
A) C5H5
B) C7H14
C) C4H7
D) C10H18
E) C4H10
A) C5H5
B) C7H14
C) C4H7
D) C10H18
E) C4H10

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following could be the molecular formula for an alkene?
A) C7H7
B) C8H16
C) C2H3
D) C2H2
E) C2H6
A) C7H7
B) C8H16
C) C2H3
D) C2H2
E) C2H6

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
26
How many constitutional isomers that are dibromocyclopropanes exist?
A) 3
B) 8
C) 4
D) 2
E) 5
A) 3
B) 8
C) 4
D) 2
E) 5

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following could be the molecular formula for an alkane?
A) C4H4
B) C7H14
C) C3H5
D) C9H16
E) C5H12
A) C4H4
B) C7H14
C) C3H5
D) C9H16
E) C5H12

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
28
Compounds that have the same molecular formula but different structural formulas are called structural, or constitutional,
A) isomers.
B) derivatives.
C) congeners.
D) isotopes.
E) progeners.
A) isomers.
B) derivatives.
C) congeners.
D) isotopes.
E) progeners.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
29
How many constitutional isomers that are trifluoroethanes exist?
A) 3
B) 2
C) 4
D) 8
E) 5
A) 3
B) 2
C) 4
D) 8
E) 5

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following could be the molecular formula for an alkene?
A) C9H9
B) C6H12
C) C9H17
D) C5H8
E) C2H6
A) C9H9
B) C6H12
C) C9H17
D) C5H8
E) C2H6

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following could be the molecular formula for an alkene?
A) C5H5
B) C2H4
C) C3H5
D) C9H16
E) C4H10
A) C5H5
B) C2H4
C) C3H5
D) C9H16
E) C4H10

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
32
1-Chlorobutane and 2-chlorobutane are
A) isomers.
B) the same compound.
C) neither isomers nor the same compound.
A) isomers.
B) the same compound.
C) neither isomers nor the same compound.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
33
Which pair are actually the same compound?
A) (CH3)3 N and CH3CH2CH2NH2
B) CH3CH2CH(NH2)CH3 and CH3CH(NH2)CH2CH3
C) CH3CH(Cl)CH3 and CH3CH2CH(Cl)CH3
D) CH3(CH2)2CH3 and CH3CH2CH(CH3)2
E) CH3CH2CH2NH2 and CH3CH2CH2N2H2
A) (CH3)3 N and CH3CH2CH2NH2
B) CH3CH2CH(NH2)CH3 and CH3CH(NH2)CH2CH3
C) CH3CH(Cl)CH3 and CH3CH2CH(Cl)CH3
D) CH3(CH2)2CH3 and CH3CH2CH(CH3)2
E) CH3CH2CH2NH2 and CH3CH2CH2N2H2

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
34
Which organic compound is represented by a triangle?
A) Trianol
B) Cyclopropane
C) Benzene
D) Cyclobutane
E) Methane
A) Trianol
B) Cyclopropane
C) Benzene
D) Cyclobutane
E) Methane

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following could be the molecular formula for an alkene?
A) C8H8
B) C9H18
C) C5H9
D) C5H8
E) C8H18
A) C8H8
B) C9H18
C) C5H9
D) C5H8
E) C8H18

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
36
Which pair are isomers?
A) CH3 CH2 CH(NH2) CH3 and CH3 CH(NH2) CH2 CH3
B) CH3CH(Cl)CH3 and CH3CH2CH(Cl)CH3
C) (CH3)3 N and CH3CH2CH2NH2
D) CH3 (CH2)2 CH3 and CH3 CH2CH(CH3)2
E) CH3CH2CH(Cl)CH3 and CH3 CH(NH2) CH2 CH3
A) CH3 CH2 CH(NH2) CH3 and CH3 CH(NH2) CH2 CH3
B) CH3CH(Cl)CH3 and CH3CH2CH(Cl)CH3
C) (CH3)3 N and CH3CH2CH2NH2
D) CH3 (CH2)2 CH3 and CH3 CH2CH(CH3)2
E) CH3CH2CH(Cl)CH3 and CH3 CH(NH2) CH2 CH3

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following could be the molecular formula for an alkane?
A) C7H7
B) C6H12
C) C5H9
D) C9H16
E) C3H8
A) C7H7
B) C6H12
C) C5H9
D) C9H16
E) C3H8

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
38
When hydrogen reacts with ethene, what is the product?
A) Cycloethene
B) Ethane
C) Propene
D) Ethyne
E) Methane
A) Cycloethene
B) Ethane
C) Propene
D) Ethyne
E) Methane

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
39
How many constitutional isomers that are dibromoethanes exist?
A) 3
B) 4
C) 5
D) 2
E) 6
A) 3
B) 4
C) 5
D) 2
E) 6

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following could be the molecular formula for an alkane?
A) C3H3
B) C7H14
C) C4H7
D) C10H18
E) C6H14
A) C3H3
B) C7H14
C) C4H7
D) C10H18
E) C6H14

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
41
Alkyl halides are distinguished by the fact that at least one hydrogen atom in a hydrocarbon has been replaced by a(n)
A) alkyl group.
B) OH group.
C) halogen atom.
D) oxygen atom.
E) CH3 group.
A) alkyl group.
B) OH group.
C) halogen atom.
D) oxygen atom.
E) CH3 group.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
42
When a carboxylic acid reacts with an amine, the products are water and a(n)
A) aldehyde.
B) ester.
C) amide.
D) salt.
E) ketone.
A) aldehyde.
B) ester.
C) amide.
D) salt.
E) ketone.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
43
1-Bromobutane and 4-bromobutane are
A) the same compound.
B) neither isomers nor the same compound.
C) isomers.
A) the same compound.
B) neither isomers nor the same compound.
C) isomers.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
44
A hydrocarbon that contains one or more benzene rings must be classified as
A) an alkane.
B) a cycloalkane.
C) aromatic.
D) an alkene.
E) an alkyne.
A) an alkane.
B) a cycloalkane.
C) aromatic.
D) an alkene.
E) an alkyne.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
45
Ethene and ethane are
A) isomers.
B) the same compound.
C) neither isomers nor the same compound.
A) isomers.
B) the same compound.
C) neither isomers nor the same compound.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
46
4-Fluoroheptane and 3-fluoroheptane are
A) isomers.
B) the same compound.
C) neither isomers nor the same compound.
A) isomers.
B) the same compound.
C) neither isomers nor the same compound.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
47
Cholesterol belongs to what class of organic compounds?
A) Alkyl halides
B) Esters
C) Carboxylic acids
D) Alcohols
A) Alkyl halides
B) Esters
C) Carboxylic acids
D) Alcohols

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
48
Methyl salicylate is an ester commonly called
A) glycerine.
B) oil of wintergreen.
C) heroin.
D) chloroform.
E) methamphetamine.
A) glycerine.
B) oil of wintergreen.
C) heroin.
D) chloroform.
E) methamphetamine.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
49
A hydrocarbon that is not classified as aromatic must be classified as
A) nucleophilic.
B) synthetic.
C) aliphatic.
D) carboxylic.
A) nucleophilic.
B) synthetic.
C) aliphatic.
D) carboxylic.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
50
2-Bromobutane and 4-bromobutane are
A) the same compound.
B) neither isomers nor the same compound.
C) isomers.
A) the same compound.
B) neither isomers nor the same compound.
C) isomers.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
51
Alcohols are distinguished by the fact that at least one hydrogen atom in a hydrocarbon has been replaced by a(n)
A) carboxyl group.
B) alkyl group.
C) hydroxyl group.
D) oxygen atom.
A) carboxyl group.
B) alkyl group.
C) hydroxyl group.
D) oxygen atom.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
52
CH3 CONHCH2 CH3 is an example of a(n)
A) amide.
B) amine.
C) ester.
D) carboxylic acid.
A) amide.
B) amine.
C) ester.
D) carboxylic acid.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
53
What organic compound is represented by a hexagon with a circle inside?
A) Cyclohexane
B) Cycloheptane
C) Benzene
D) Ethanol
E) Methane
A) Cyclohexane
B) Cycloheptane
C) Benzene
D) Ethanol
E) Methane

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
54
When a carboxylic acid reacts with an alcohol, the products are water and a(n)
A) aldehyde.
B) ester.
C) salt.
D) amide.
E) ketone.
A) aldehyde.
B) ester.
C) salt.
D) amide.
E) ketone.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
55
Chlorine is not present in
A) CFCs.
B) chloroform.
C) Freon-12.
D) Teflon.
A) CFCs.
B) chloroform.
C) Freon-12.
D) Teflon.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
56
What is the molecular formula for benzene?
A) C6H6
B) C6H12
C) C6H14
D) C6H8
E) None of these
A) C6H6
B) C6H12
C) C6H14
D) C6H8
E) None of these

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
57
The most common aromatic hydrocarbon is a clear, colorless liquid named
A) naphthalene.
B) hexane.
C) benzene.
D) ethanol.
E) methane.
A) naphthalene.
B) hexane.
C) benzene.
D) ethanol.
E) methane.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
58
CFCs are
A) alcohols.
B) hydrocarbons.
C) alkyl halides.
D) amides.
A) alcohols.
B) hydrocarbons.
C) alkyl halides.
D) amides.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
59
A solution of 5% acetic acid and 95% water is called
A) chloroform.
B) antifreeze.
C) vinegar.
D) gasohol.
E) alcohol.
A) chloroform.
B) antifreeze.
C) vinegar.
D) gasohol.
E) alcohol.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
60
Acetic acid consists of a(n) ______________ group attached to a carboxyl group.
A) amino
B) methyl
C) hydroxyl
D) ethyl
A) amino
B) methyl
C) hydroxyl
D) ethyl

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
61
Which alcohol is used as an antifreeze?
A) 2-Hydroxypropane
B) Ethylene glycol
C) Ethanol
D) Methanol
A) 2-Hydroxypropane
B) Ethylene glycol
C) Ethanol
D) Methanol

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
62
We get sucrose and water if we chemically combine
A) fructose and maltose.
B) glucose and fructose.
C) maltose and lactose.
D) glucose and lactose.
A) fructose and maltose.
B) glucose and fructose.
C) maltose and lactose.
D) glucose and lactose.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
63
Which alcohol is used in rubbing alcohol?
A) 2-Hydroxypropane
B) Ethylene glycol
C) Ethanol
D) Methanol
A) 2-Hydroxypropane
B) Ethylene glycol
C) Ethanol
D) Methanol

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
64
Another name for glucose is
A) dextrose.
B) malt sugar.
C) sucrose.
D) lactose.
A) dextrose.
B) malt sugar.
C) sucrose.
D) lactose.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
65
Which sugar is found in greater than normal amounts in the blood of a diabetic?
A) Sucrose
B) Lactose
C) Glucose
D) Fructose
A) Sucrose
B) Lactose
C) Glucose
D) Fructose

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
66
Which simplest alcohol is used in race cars as fuel?
A) 2-Propanol
B) Ethylene glycol
C) Ethanol
D) Methanol
A) 2-Propanol
B) Ethylene glycol
C) Ethanol
D) Methanol

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
67
An advantage of synthetic detergents over soaps is that they
A) do not contain sulfur.
B) dissolve grease.
C) do not form scum in hard water.
D) are more readily biodegradable.
A) do not contain sulfur.
B) dissolve grease.
C) do not form scum in hard water.
D) are more readily biodegradable.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is not a carbohydrate?
A) Maltose
B) Glucose
C) Fructose
D) Glyceryl tristearate
A) Maltose
B) Glucose
C) Fructose
D) Glyceryl tristearate

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is a carbohydrate?
A) Maltose
B) Glycine
C) Ribonucleic acid
D) Glyceryl tristearate
A) Maltose
B) Glycine
C) Ribonucleic acid
D) Glyceryl tristearate

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following is not a carbohydrate?
A) Maltose
B) Glycine
C) Fructose
D) Glucose
A) Maltose
B) Glycine
C) Fructose
D) Glucose

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
71
The general formula RNH2 is that of
A) an ester.
B) an amine.
C) an amide.
D) none of these
A) an ester.
B) an amine.
C) an amide.
D) none of these

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
72
Which compound has three hydroxyl groups?
A) Ethylene glycol
B) Ethanol
C) Cholesterol
D) Glycerol
A) Ethylene glycol
B) Ethanol
C) Cholesterol
D) Glycerol

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
73
What are the basic building blocks of proteins?
A) Amino acids
B) Starches
C) Sugars
D) Carboxylic acids
A) Amino acids
B) Starches
C) Sugars
D) Carboxylic acids

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
74
Which sugar is a chemically bonded combination of two other sugars?
A) Sucrose
B) Lactose
C) Glucose
D) Fructose
A) Sucrose
B) Lactose
C) Glucose
D) Fructose

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
75
Triesters of long-chain acids with glycerol are called
A) condensation polymers.
B) fats.
C) soaps.
D) synthetic detergents.
A) condensation polymers.
B) fats.
C) soaps.
D) synthetic detergents.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
76
The suffix -ol in a compound's name signifies
A) an enzyme.
B) an amide.
C) an alcohol.
D) a carbohydrate.
A) an enzyme.
B) an amide.
C) an alcohol.
D) a carbohydrate.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
77
Starch is a polymer that consists of long chains of ______________ units.
A) lactose
B) fructose
C) maltose
D) glucose
A) lactose
B) fructose
C) maltose
D) glucose

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
78
The suffix -ose in a compound's name signifies a(n)
A) amide.
B) alcohol.
C) carbohydrate.
D) enzyme.
A) amide.
B) alcohol.
C) carbohydrate.
D) enzyme.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
79
Which sugar is the sweetest of all sugars?
A) Sucrose
B) Lactose
C) Glucose
D) Fructose
A) Sucrose
B) Lactose
C) Glucose
D) Fructose

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
80
Which alcohol is used in beverages?
A) 2-Propanol
B) Ethylene glycol
C) Ethanol
D) Methanol
A) 2-Propanol
B) Ethylene glycol
C) Ethanol
D) Methanol

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck



