Deck 5: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/64
Play
Full screen (f)
Deck 5: Nuclear Chemistry
1
When a positron is emitted from the nucleus of an atom, the mass number
A)decreases by two units.
B)remains the same.
C)increases by two units.
D)decreases by one unit.
E)increases by one unit.
A)decreases by two units.
B)remains the same.
C)increases by two units.
D)decreases by one unit.
E)increases by one unit.
remains the same.
2
What is the nuclear symbol for a radioactive isotope of copper with a mass number of 60 ?
A)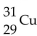
B)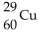
C)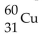
D) 29Cu
E)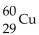
A)
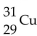
B)
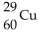
C)
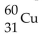
D) 29Cu
E)
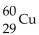
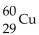
3
The nuclar reaction shown below is an example of what type of process? 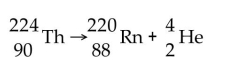
A)alpha decay
B)translation
C)fission
D)beta decay
E)fusion

A)alpha decay
B)translation
C)fission
D)beta decay
E)fusion
alpha decay
4
The process in which a nucleus spontaneously breaks down by emitting radiation is known as
A)transmutation.
B)radioactive decay.
C)fusion.
D)transformation.
E)a chain reaction.
A)transmutation.
B)radioactive decay.
C)fusion.
D)transformation.
E)a chain reaction.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
5
If absorbed internally, alpha particle emitters are the most damaging because alpha particles
A)have the greatest mass.
B)consist of high energy electrons.
C)have the greatest energy.
D)consist of pure energy.
E)have the largest charge.
A)have the greatest mass.
B)consist of high energy electrons.
C)have the greatest energy.
D)consist of pure energy.
E)have the largest charge.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
6
When a gamma ray is emitted from the nucleus of an atom, the nuclear mass
A)decreases by two units.
B)decreases by one unit.
C)increases by two units.
D)increases by one unit.
E)remains the same.
A)decreases by two units.
B)decreases by one unit.
C)increases by two units.
D)increases by one unit.
E)remains the same.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
7
When an alpha particle is emitted from the nucleus of an atom, the nuclear mass
A)increases by two units.
B)decreases by two units.
C)increases by one unit.
D)decreases by four units.
E)remains the same.
A)increases by two units.
B)decreases by two units.
C)increases by one unit.
D)decreases by four units.
E)remains the same.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
8
Which is NOT a way to minimize your exposure to radiation?
A)staying a longer time
B)wearing a lead apron
C)keeping a good distance
D)wearing lead-lined gloves
E)standing behind a thick concrete wall
A)staying a longer time
B)wearing a lead apron
C)keeping a good distance
D)wearing lead-lined gloves
E)standing behind a thick concrete wall

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
9
The symbol 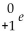 is a symbol used for a(n)
is a symbol used for a(n)
A)beta particle.
B)positron.
C)alpha particle.
D)gamma ray.
E)proton.
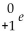 is a symbol used for a(n)
is a symbol used for a(n)A)beta particle.
B)positron.
C)alpha particle.
D)gamma ray.
E)proton.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
10
A positron is a particle emitted from the nucleus that has the same mass as a(n)
A)proton emitted from the nucleus.
B)beta particle.
C)electron but has a positive charge.
D)neutron but has a positive charge.
E)alpha particle.
A)proton emitted from the nucleus.
B)beta particle.
C)electron but has a positive charge.
D)neutron but has a positive charge.
E)alpha particle.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
11
The nuclear symbol of helium, 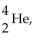 is also the symbol for a(n)
is also the symbol for a(n)
A)alpha particle.
B)proton.
C)neutron.
D)beta particle.
E)gamma ray.
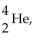 is also the symbol for a(n)
is also the symbol for a(n)A)alpha particle.
B)proton.
C)neutron.
D)beta particle.
E)gamma ray.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
12
A nuclear equation is balanced when
A)the charges of the particles and atoms are the same on both sides of the equation.
B)the same elements are found on both sides of the equation.
C)the same particles and atoms are on both sides of the equation.
D)different particles and atoms are on both sides of the equation.
E)the sum of the mass numbers and the sum of the atomic numbers of the particles and atoms are the same on both sides of the equation.
A)the charges of the particles and atoms are the same on both sides of the equation.
B)the same elements are found on both sides of the equation.
C)the same particles and atoms are on both sides of the equation.
D)different particles and atoms are on both sides of the equation.
E)the sum of the mass numbers and the sum of the atomic numbers of the particles and atoms are the same on both sides of the equation.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
13
Gamma rays require the heaviest shielding of all the common types of nuclear radiation because gamma rays have the
A)lowest energy.
B)most intense color.
C)largest particles.
D)heaviest particles.
E)highest energy.
A)lowest energy.
B)most intense color.
C)largest particles.
D)heaviest particles.
E)highest energy.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is suitable as a minimum shielding for beta particles?
A)1 m of water
B)1 m of concrete
C)gloves
D)air
E)1 cm of lead
A)1 m of water
B)1 m of concrete
C)gloves
D)air
E)1 cm of lead

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
15
In the nuclear equation of a beta emitter,
A)the new nucleus contains 1 less proton.
B)the new nucleus contains 2 fewer protons.
C)the mass number of the new nucleus is 4 less than that of the original nucleus.
D)the new nucleus contains 2 more protons.
E)the new nucleus contains 1 more proton.
A)the new nucleus contains 1 less proton.
B)the new nucleus contains 2 fewer protons.
C)the mass number of the new nucleus is 4 less than that of the original nucleus.
D)the new nucleus contains 2 more protons.
E)the new nucleus contains 1 more proton.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
16
The symbol 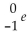 is a symbol used for a(n)
is a symbol used for a(n)
A)alpha particle.
B)proton.
C)beta particle.
D)neutron.
E)gamma ray.
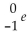 is a symbol used for a(n)
is a symbol used for a(n)A)alpha particle.
B)proton.
C)beta particle.
D)neutron.
E)gamma ray.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
17
For 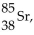 there are
there are
A) 38 protons and 47 neutrons.
B) 85 protons and 47 neutrons.
C) 47 protons and 38 neutrons.
D) 85 protons and 38 neutrons.
E) 38 protons and 85 neutrons.
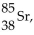 there are
there areA) 38 protons and 47 neutrons.
B) 85 protons and 47 neutrons.
C) 47 protons and 38 neutrons.
D) 85 protons and 38 neutrons.
E) 38 protons and 85 neutrons.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
18
The damaging effects of radiation on the body are a result of
A)the production of radioactive sodium ions in the body.
B)the formation of unstable ions.
C)transmutation reactions in the body.
D)extensive damage to nerve cells.
E)the formation of radioactive atoms in the body.
A)the production of radioactive sodium ions in the body.
B)the formation of unstable ions.
C)transmutation reactions in the body.
D)extensive damage to nerve cells.
E)the formation of radioactive atoms in the body.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following types of radiation has the highest energy?
A)gamma rays
B)visible light
C)alpha particles
D)beta particles
E)All of these have the same energy.
A)gamma rays
B)visible light
C)alpha particles
D)beta particles
E)All of these have the same energy.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
20
The product from the alpha decay of 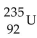 is
is
A)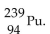
B)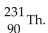
C)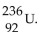
D)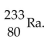
Е)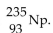
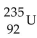 is
isA)
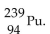
B)
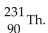
C)
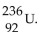
D)
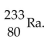
Е)
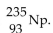

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
21
A patient receives 10 mrads of gamma radiation. If the factor that adjusts for biological damage for for gamma radiation is 1, how many mrems did the patient receive?
A)10 mrem
B)200 mrem
C)20 mrem
D)2 mrem
E)5 mrem
A)10 mrem
B)200 mrem
C)20 mrem
D)2 mrem
E)5 mrem

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
22
The unit used to measure the amount of radiation absorbed by a gram of material is called the
A)RBE.
B)rem.
C)curie.
D)MPD.
E)rad.
A)RBE.
B)rem.
C)curie.
D)MPD.
E)rad.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
23
What particle is emitted in the following nuclear reaction? 
A) beta particle
B) alpha particle
C) gamma ray
D) neutron
E) proton

A) beta particle
B) alpha particle
C) gamma ray
D) neutron
E) proton

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
24
A sample of technetium-99m has an activity of 1.5 Ci. How many disintegrations occur in the technetium-99m sample in 5.0 sec?
A)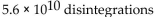
B)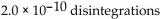
C)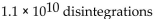
D)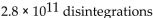
E)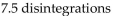
A)
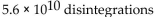
B)
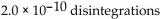
C)
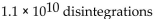
D)
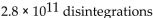
E)
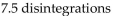

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
25
Iodine-131 decays by beta decay to
A)iodine-132.
B)xenon-131.
C)tellurium-131.
D)bromine-131.
E)iodine-130.
A)iodine-132.
B)xenon-131.
C)tellurium-131.
D)bromine-131.
E)iodine-130.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
26
Radium-226 decays by alpha decay to
A)barium-131.
B)cobalt-60.
C)radon-222.
D)polonium-218.
E)carbon-14.
A)barium-131.
B)cobalt-60.
C)radon-222.
D)polonium-218.
E)carbon-14.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
27
One symptom of mild radiation sickness is
A)a raised white cell count.
B)a raised red blood cell count.
C)a lowered white cell count.
D)a lowered red blood cell count.
E)a white cell count of zero.
A)a raised white cell count.
B)a raised red blood cell count.
C)a lowered white cell count.
D)a lowered red blood cell count.
E)a white cell count of zero.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
28
When aluminum-27 is bombarded with a neutron, a gamma ray is emitted. What radioactive isotope is produced?
A)aluminum-28
B)magnesium-28
C)magnesium-27
D)silicon-28
E)silicon-27
A)aluminum-28
B)magnesium-28
C)magnesium-27
D)silicon-28
E)silicon-27

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
29
What is missing in the nuclear reaction shown below? 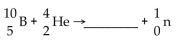
A) a neutron
B)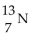
C)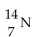
D)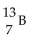
E)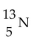

A) a neutron
B)
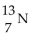
C)
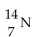
D)
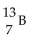
E)
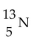

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
30
The recommended dosage of I-131 for a test is 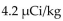 of body weight. How many mCi should be given to a 55 kg patient?
of body weight. How many mCi should be given to a 55 kg patient? 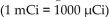
A) 13.8 mCi
B) 0.076 mCi
C) 230 mCi
D) 760 mCi
E) 0.23 mCi
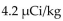 of body weight. How many mCi should be given to a 55 kg patient?
of body weight. How many mCi should be given to a 55 kg patient? 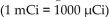
A) 13.8 mCi
B) 0.076 mCi
C) 230 mCi
D) 760 mCi
E) 0.23 mCi

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
31
What particle is emitted in the following nuclear reaction?
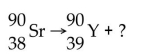
A)beta particle
B)gamma ray
C)neutron
D)proton
E)alpha particle
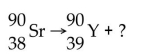
A)beta particle
B)gamma ray
C)neutron
D)proton
E)alpha particle

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
32
What is missing in the nuclear reaction shown below?
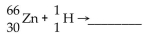
A)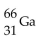
B)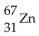
C)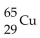
D)
E) a proton
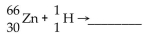
A)
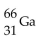
B)
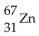
C)
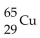
D)

E) a proton

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
33
Nitrogen-17 is a beta emitter. What is the isotope produced by the radioactive decay?
A)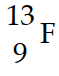
B)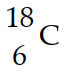
C)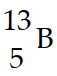
D)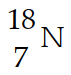
E)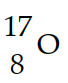
A)
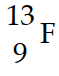
B)
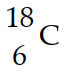
C)
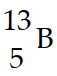
D)
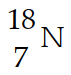
E)
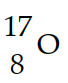

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
34
Why is it important that radioisotopes used in diagnostic tests have short half-lives?
A)These radioisotopes are less expensive.
B)These radioisotopes are more abundant in nature.
C)These radioisotopes have a greater activity so they are easier to monitor.
D)This is necessary so the radioisotopes will have high energy.
E)This minimizes the harmful side effects of the radiation.
A)These radioisotopes are less expensive.
B)These radioisotopes are more abundant in nature.
C)These radioisotopes have a greater activity so they are easier to monitor.
D)This is necessary so the radioisotopes will have high energy.
E)This minimizes the harmful side effects of the radiation.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
35
What is the radiation particle used in the bombardment of nitrogen-14? 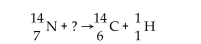
A)neutron
B)gamma ray
C)alpha particle
D)beta particle
E)proton
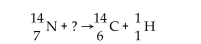
A)neutron
B)gamma ray
C)alpha particle
D)beta particle
E)proton

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
36
A sample of cerium-141 for a diagnostic test was dissolved in saline solution to an activity of 4.5 mCi/mL. If the patient undergoing the test needs a dose of 10. mCi, how much of the solution should be injected into the
Patient?
A).45 mL
B)2.2 mL
C)4.5 mL
D)22 mL
E)45 mL
Patient?
A).45 mL
B)2.2 mL
C)4.5 mL
D)22 mL
E)45 mL

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
37
A person begins to suffer radiation sickness at an exposure level of
A)600 rem.
B)25 rem.
C)100 rem.
D)5 rem.
E)500 rem.
A)600 rem.
B)25 rem.
C)100 rem.
D)5 rem.
E)500 rem.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
38
A patient receives 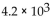 mrads of iodine-131, which emits
mrads of iodine-131, which emits 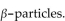 If the factor that adjusts for biological damage is 1 for beta particles, how many rems did the patient receive?
If the factor that adjusts for biological damage is 1 for beta particles, how many rems did the patient receive?
A) 2.0
B) 4.0
C) 40
D) 0.40
E) 0.30
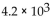 mrads of iodine-131, which emits
mrads of iodine-131, which emits 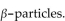 If the factor that adjusts for biological damage is 1 for beta particles, how many rems did the patient receive?
If the factor that adjusts for biological damage is 1 for beta particles, how many rems did the patient receive?A) 2.0
B) 4.0
C) 40
D) 0.40
E) 0.30

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
39
The nuclear reaction 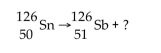 is an example of
is an example of
A)fission.
B)fusion.
C)beta decay.
D)alpha decay.
E)translation.
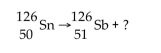 is an example of
is an example ofA)fission.
B)fusion.
C)beta decay.
D)alpha decay.
E)translation.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
40
What is missing in the nuclear reaction shown below? 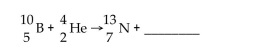
A)a neutron
B)gamma radiation
C)an alpha particle
D)a beta particle
E)a positron
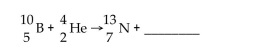
A)a neutron
B)gamma radiation
C)an alpha particle
D)a beta particle
E)a positron

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
41
An imaging technique in which a computer monitors the degree of absorption of X-ray beams is known as
A)magnetic resonance imaging (MRI).
B)radioactive iodine uptake (RAIU).
C)positron emission tomography (PET).
D)a scan.
E)computed tomography (CT).
A)magnetic resonance imaging (MRI).
B)radioactive iodine uptake (RAIU).
C)positron emission tomography (PET).
D)a scan.
E)computed tomography (CT).

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
42
Sodium-24 has a half-life of 15 hours. How many hours is three half-lives?
A)15 hours
B)45 hours
C)7.5 hours
D)30 hours
E)60 hours
A)15 hours
B)45 hours
C)7.5 hours
D)30 hours
E)60 hours

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
43
Write the word or phrase that best completes each statement or answers the question.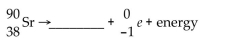
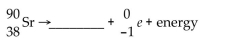

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
44
An imaging technique that detects the energy emitted by hydrogen atoms in a magnetic field is known as
A)magnetic resonance imaging (MRI).
B)radioactive tracer study.
C)computed tomography (CT).
D)positron emission tomography (PET).
E)supermagnetic tomography (SMT).
A)magnetic resonance imaging (MRI).
B)radioactive tracer study.
C)computed tomography (CT).
D)positron emission tomography (PET).
E)supermagnetic tomography (SMT).

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
45
In the Sun, nuclei of hydrogen combine to form a larger nucleus and release a great amount of energy. The process is known as
A)metathesis.
B)fusion.
C)fission.
D)ionization.
E)chain reaction.
A)metathesis.
B)fusion.
C)fission.
D)ionization.
E)chain reaction.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
46
The common unit of radioactivity which is used to measure the biological damage is the ________.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
47
The half-life of bromine-74 is 25 min. How much of a 4.0 mg sample is still active after 75 min?
A)0.25 mg
B)0.50 mg
C)4.0 mg
D)1.0 mg
E)2.0 mg
A)0.25 mg
B)0.50 mg
C)4.0 mg
D)1.0 mg
E)2.0 mg

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
48
The half-life of a radioisotope is
A)the time it takes for the radioisotope to become an isotope with one-half the atomic number of the original radioisotope.
B)the time it takes for one-half of the sample to decay.
C)the time it takes for the radioisotope to become an isotope with one-half of the atomic weight of the original radioisotope.
D)the time it takes for the radioisotope to lose one-half of its neutrons.
E)one-half of the time it takes for the radioisotope to completely decay to a nonradioactive isotope.
A)the time it takes for the radioisotope to become an isotope with one-half the atomic number of the original radioisotope.
B)the time it takes for one-half of the sample to decay.
C)the time it takes for the radioisotope to become an isotope with one-half of the atomic weight of the original radioisotope.
D)the time it takes for the radioisotope to lose one-half of its neutrons.
E)one-half of the time it takes for the radioisotope to completely decay to a nonradioactive isotope.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
49
A patient receives 3.0 mL of a solution containing technetium- 99m for a breast image. If the activity of the technetium -99m is 9.5 mCi/mL , what is the dose received by the patient?
A) 3.2 mCi
B)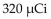
C) 29 mCi
D) 28.5 mCi
E) 9.5 mCi
A) 3.2 mCi
B)
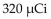
C) 29 mCi
D) 28.5 mCi
E) 9.5 mCi

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
50
The most widely used medical isotope in nuclear medicine is
A)I-131.
B)I-125.
C)P-32.
D)Co-60.
E)Tc-99m.
A)I-131.
B)I-125.
C)P-32.
D)Co-60.
E)Tc-99m.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
51
The dosage of technetium-99m for myocardial imaging is 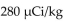 of body weight. How many mCi should be given to a patient weighing 65 kg?
of body weight. How many mCi should be given to a patient weighing 65 kg? 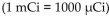
A)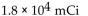
B) 18 mCi
C) 0.0043 mCi
D) 230 mCi
E) 4.3 mCi
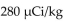 of body weight. How many mCi should be given to a patient weighing 65 kg?
of body weight. How many mCi should be given to a patient weighing 65 kg? 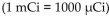
A)
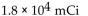
B) 18 mCi
C) 0.0043 mCi
D) 230 mCi
E) 4.3 mCi

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
52
Write the word or phrase that best completes each statement or answers the question.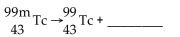
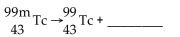

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
53
A wooden object from a prehistoric site has a carbon-14 activity of 10 counts per minute (cpm)compared to 40 cpm for new wood. If carbon-14 has a half-life of 5730 years, what is the age of the wood?
A)22,900 yr
B)1430 yr
C)17,200 yr
D)5730 yr
E)11,500 yr
A)22,900 yr
B)1430 yr
C)17,200 yr
D)5730 yr
E)11,500 yr

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
54
A sample of phosphorus-32 with an activity of 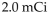 produces
produces  disintegrations per second.
disintegrations per second.
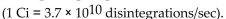
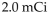 produces
produces  disintegrations per second.
disintegrations per second.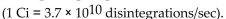

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
55
Krypton-79 has a half-life of 35 hours. How many half-lives have passed after 105 hours?
A)1 half-life
B)2 half-lives
C)3 half-lives
D)4 half-lives
E)5 half-lives
A)1 half-life
B)2 half-lives
C)3 half-lives
D)4 half-lives
E)5 half-lives

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
56
One symbol for the beta particle β. Another symbol for the same particle is ________.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
57
When an atom of uranium-235 is bombarded with neutrons, it splits into smaller nuclei and produces a great amount of energy. This nuclear process is called
A)fusion.
B)ionization.
C)decomposition.
D)chain reaction.
E)fission.
A)fusion.
B)ionization.
C)decomposition.
D)chain reaction.
E)fission.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
58
The radiation dose required to produce death in one-half of the exposed subject animals is termed the ________.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
59
Iodine-123, which is used for diagnostic imaging in the thyroid, has a half-life of 13 hours. If 50.0 mg of I-123 were prepared at 8:00 A.M. on Monday, how many mg remain at 10:00 A.M. on the following day?
A)3.13 mg
B)6.25 mg
C)25.0 mg
D)12.5 mg
E)50.0 mg
A)3.13 mg
B)6.25 mg
C)25.0 mg
D)12.5 mg
E)50.0 mg

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
60
Write the word or phrase that best completes each statement or answers the question.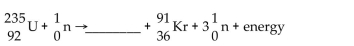
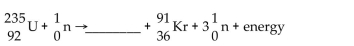

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
61
The diagnostic imaging technique that depends on magnetic fields and radio waves, not radioactivity, is called
________.
________.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
62
The radioisotope used as a diagnostic tool to measure thyroid function is ________.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
63
The time needed for a radioactive sample to decay to one-half of its original activity is called the ________.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
64
The process by which a large nucleus breaks into smaller pieces, releasing large amounts of energy is called
nuclear ________.
nuclear ________.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck



