Deck 2: Alkanes: the Nature of Organic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 2: Alkanes: the Nature of Organic Compounds
1
Provide a skeletal structure for 5-tert-butyl-2,3-dimethyloctane.
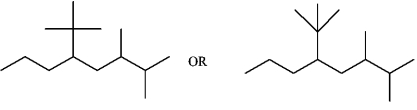
2
How many constitutional isomers are there with the molecular formula C6H14?
A) 3
B) 4
C) 5
D) 8
A) 3
B) 4
C) 5
D) 8
5
3
Which hydrogen atom(s) in the following compound is (are) classified as tertiary? 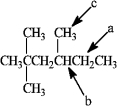
A) a
B) b
C) c
D) both a and c
E) There are no tertiary hydrogen atoms.

A) a
B) b
C) c
D) both a and c
E) There are no tertiary hydrogen atoms.
both a and c
4
Provide a proper IUPAC name for the compound given below. 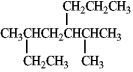


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
Name these groups (left to right). 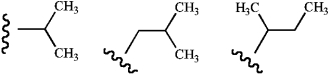 where
where  represents the parent chain.
represents the parent chain.
A) sec-propyl, sec-butyl, isobutyl
B) isopropyl, isobutyl, sec-butyl
C) sec-propyl, tert-butyl, isobutyl
D) isopropyl, tert-butyl, isobutyl
E) isopropyl, tert-butyl, sec-butyl
 where
where  represents the parent chain.
represents the parent chain.A) sec-propyl, sec-butyl, isobutyl
B) isopropyl, isobutyl, sec-butyl
C) sec-propyl, tert-butyl, isobutyl
D) isopropyl, tert-butyl, isobutyl
E) isopropyl, tert-butyl, sec-butyl

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
What is the IUPAC name of the following compound? 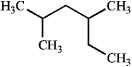
A) 2-ethyl-4-methylpentane
B) 2,4-dimethylhexane
C) 3,5-dimethylhexane
D) 1,1,3-trimethylpentane

A) 2-ethyl-4-methylpentane
B) 2,4-dimethylhexane
C) 3,5-dimethylhexane
D) 1,1,3-trimethylpentane

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
Provide the proper IUPAC name for the compound below. 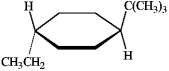


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
Designate each carbon as primary, secondary, tertiary, or quaternary. 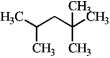


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
Instructions: Refer to the structure below to answer the following question(s). 
Refer to instructions. Which groups have a 1,3-diaxial interaction with each other?

Refer to instructions. Which groups have a 1,3-diaxial interaction with each other?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
Draw and name the seven constitutional isomers for cycloalkane, C6H12.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
To which functional group classification does the following molecule belong? 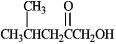
A) ester
B) ketone
C) alcohol
D) carboxylic acid
E) both b and c
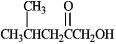
A) ester
B) ketone
C) alcohol
D) carboxylic acid
E) both b and c

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following alkanes is the most likely to be a liquid at room temperature?
A) propane
B) butane
C) pentane
D) hexane
A) propane
B) butane
C) pentane
D) hexane

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following functional group classifications do not contain oxygen?
A) ether
B) thiol
C) aldehyde
D) ester
E) amide
A) ether
B) thiol
C) aldehyde
D) ester
E) amide

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
Instructions: Refer to the structure below to answer the following question(s). 
Refer to instructions. Which of the labeled groups in the structure are equatorial?

Refer to instructions. Which of the labeled groups in the structure are equatorial?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
One of the functional group classifications is characterized by the presence of an sp2 hybridized carbon atom. This functional group could be:
A) alkyl halide
B) sulfide
C) alcohol
D) aldehyde
E) alkyne
A) alkyl halide
B) sulfide
C) alcohol
D) aldehyde
E) alkyne

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
Instructions: Refer to the structure below to answer the following question(s). 
Refer to instructions. Which of the labeled groups is trans to b?

Refer to instructions. Which of the labeled groups is trans to b?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
Circle and name each functional group in the following structure. 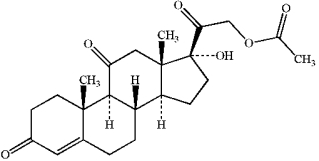 cortisone acetate (active ingredient in steroid skin cream)
cortisone acetate (active ingredient in steroid skin cream)
 cortisone acetate (active ingredient in steroid skin cream)
cortisone acetate (active ingredient in steroid skin cream)
Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
The carbon atoms within the functional group of the following classifications are: alkene ester carboxylic acid amide
A) sp
B) sp2
C) sp3
D) sp2 and sp3
A) sp
B) sp2
C) sp3
D) sp2 and sp3

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
Draw the structure of a five carbon ketone containing one tertiary carbon atom.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
Label the following pair of compounds as: 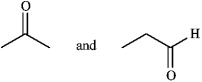
A) identical
B) constitutional isomers
C) stereoisomers
D) unrelated

A) identical
B) constitutional isomers
C) stereoisomers
D) unrelated

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
Instructions:
MATCH a structure below to each of the following descriptions and place the letter corresponding to the structure in the blank.
Refer to Instructions. _______is an amino aldehyde.
A)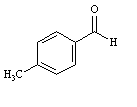
B)
C)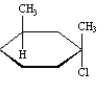
D)
E)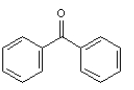
F)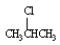
G)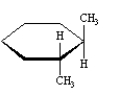
MATCH a structure below to each of the following descriptions and place the letter corresponding to the structure in the blank.
Refer to Instructions. _______is an amino aldehyde.
A)

B)

C)

D)

E)

F)
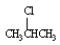
G)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
Draw a structure corresponding to the following name:
cis-1-sec-butyl-2-ethylcyclopentane
cis-1-sec-butyl-2-ethylcyclopentane

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
Draw two isomeric alcohols with the formula C4H10O.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following structures represents trans-1,2-dimethylcyclohexane?
A)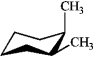
B)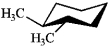
C)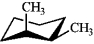
D)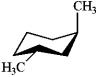
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
This skeletal structure corresponds to the molecular formula: 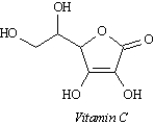
A) C5H6O6
B) C7H10O6
C) C6H6O6
D) C6H8O6

A) C5H6O6
B) C7H10O6
C) C6H6O6
D) C6H8O6

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
Substitution of which of the following groups on a cycloalkane would result in the greatest amount of steric strain?
A) bromo
B) ethyl
C) isopropyl
D) hydroxyl
A) bromo
B) ethyl
C) isopropyl
D) hydroxyl

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is the most stable conformation of trans-1-ethyl-3-methylcyclohexane?
A)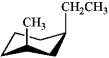
B)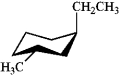
C)
D)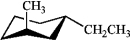
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
Instructions:
MATCH a structure below to each of the following descriptions and place the letter corresponding to the structure in the blank.
Refer to Instructions. _____is a cyclic alkane with two cis methyl groups.
A)
B)
C)
D)
E)
F)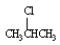
G)
MATCH a structure below to each of the following descriptions and place the letter corresponding to the structure in the blank.
Refer to Instructions. _____is a cyclic alkane with two cis methyl groups.
A)

B)

C)

D)

E)

F)
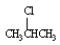
G)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
Instructions: (-)-Menthol is responsible for the characteristic flavor and taste of peppermint. The structure of (-)-menthol is shown below. Use this information to answer the following question. 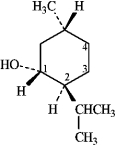
-Refer to instructions. On the chair template provided below, draw the two chair conformations that are in equilibrium for (-)-menthol.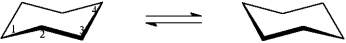

-Refer to instructions. On the chair template provided below, draw the two chair conformations that are in equilibrium for (-)-menthol.


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
Instructions: For each disubstituted cyclohexane below, draw its ring-flip isomer. Circle the most stable conformation and label the substituent groups as axial or equatorial.
Draw and label:
Draw and label:


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is the most stable conformation of cis-1-isopropyl-3-methylcyclohexane?
A)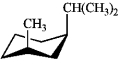
B)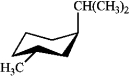
C)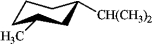
D)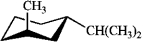
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
In which of the following compounds would the carbon-carbon bond angle diverge the greatest from 109 ?
A) cyclodecane
B) cyclooctane
C) cyclopentane
D) cyclopropane
A) cyclodecane
B) cyclooctane
C) cyclopentane
D) cyclopropane

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
Instructions: (-)-Menthol is responsible for the characteristic flavor and taste of peppermint. The structure of (-)-menthol is shown below. Use this information to answer the following question. 
-D-Pinitol is an interesting hexahydroxycyclohexane, whose structure is shown below.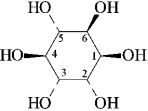 On the templates provided, draw the two chair conformations that are in equilibrium for D-pinitol. Circle the most stable conformation.
On the templates provided, draw the two chair conformations that are in equilibrium for D-pinitol. Circle the most stable conformation. 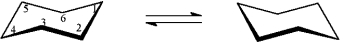

-D-Pinitol is an interesting hexahydroxycyclohexane, whose structure is shown below.
 On the templates provided, draw the two chair conformations that are in equilibrium for D-pinitol. Circle the most stable conformation.
On the templates provided, draw the two chair conformations that are in equilibrium for D-pinitol. Circle the most stable conformation. 

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
Instructions:
MATCH a structure below to each of the following descriptions and place the letter corresponding to the structure in the blank.
Refer to Instructions. _____is an aromatic ketone.
A)
B)
C)
D)
E)
F)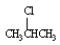
G)
MATCH a structure below to each of the following descriptions and place the letter corresponding to the structure in the blank.
Refer to Instructions. _____is an aromatic ketone.
A)

B)

C)

D)

E)

F)
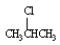
G)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
Instructions: For each disubstituted cyclohexane below, draw its ring-flip isomer. Circle the most stable conformation and label the substituent groups as axial or equatorial.
Draw and label: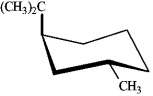
Draw and label:


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
Instructions: Label each pair of compounds below as:
A)identical
B)stereoisomers
C)constitutional isomers
D)identical, but differing in conformation
Where stereoisomers are present, label the isomers as cis and trans.
Label:
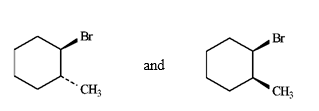
A)identical
B)stereoisomers
C)constitutional isomers
D)identical, but differing in conformation
Where stereoisomers are present, label the isomers as cis and trans.
Label:


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
Instructions:
MATCH a structure below to each of the following descriptions and place the letter corresponding to the structure in the blank.
Refer to Instructions. _____is a tertiary chloride.
A)
B)
C)
D)
E)
F)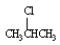
G)
MATCH a structure below to each of the following descriptions and place the letter corresponding to the structure in the blank.
Refer to Instructions. _____is a tertiary chloride.
A)

B)

C)

D)

E)

F)
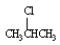
G)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following cycloalkanes has the most ring strain?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following structures represents trans-1,3-dimethylcyclohexane?
A)
B)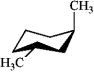
C)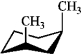
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
Instructions: Label each pair of compounds below as:
A)identical
B)stereoisomers
C)constitutional isomers
D)identical, but differing in conformation
Where stereoisomers are present, label the isomers as cis and trans.
Label:
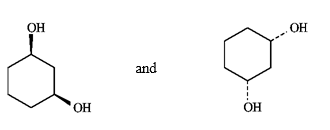
A)identical
B)stereoisomers
C)constitutional isomers
D)identical, but differing in conformation
Where stereoisomers are present, label the isomers as cis and trans.
Label:


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
Instructions:
Consider the conformations of 2-methylbutane shown below to answer the following questions.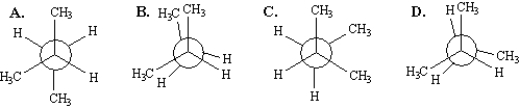
Refer to Instructions. Which of the structures represents the most stable conformation of 2-methylbutane?
Consider the conformations of 2-methylbutane shown below to answer the following questions.

Refer to Instructions. Which of the structures represents the most stable conformation of 2-methylbutane?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
Instructions:
Consider the conformations of 2-methylbutane shown below to answer the following questions.
Draw the Newman projection for the specified conformations for rotation about the C-C bond of 1,2-dichloroethane.
There are two eclipsed conformations of 1,2-dichloroethane. Draw them.
Consider the conformations of 2-methylbutane shown below to answer the following questions.

Draw the Newman projection for the specified conformations for rotation about the C-C bond of 1,2-dichloroethane.
There are two eclipsed conformations of 1,2-dichloroethane. Draw them.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
Instructions:
Consider the conformations of 2-methylbutane shown below to answer the following questions.
Draw the Newman projection for the specified conformations for rotation about the C-C bond of 1,2-dichloroethane.
There are two staggered conformations of 1,2-dichloroethane. Draw them.
Consider the conformations of 2-methylbutane shown below to answer the following questions.

Draw the Newman projection for the specified conformations for rotation about the C-C bond of 1,2-dichloroethane.
There are two staggered conformations of 1,2-dichloroethane. Draw them.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
Instructions:
Consider the conformations of 2-methylbutane shown below to answer the following questions.
Refer to Instructions. Which of the structures represents the least stable conformation of 2-methylbutane?
Consider the conformations of 2-methylbutane shown below to answer the following questions.

Refer to Instructions. Which of the structures represents the least stable conformation of 2-methylbutane?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck



