Deck 16: Biomass Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 16: Biomass Energy
1
A 1 GWe wood burning generating station operates at a Carnot efficiency 

A 1 GWe generating facility with a capacity factor of 75% will produce a total
energy per year of
energy per year of

2
Using information from Figure 16.9 and an energy content of MSW of 10
MJ/kg, estimate the number of 35% efficient 1000 MWe coal-fired power plants that could
be eliminated if 50% of MSW were burned to produce electricity.
MJ/kg, estimate the number of 35% efficient 1000 MWe coal-fired power plants that could
be eliminated if 50% of MSW were burned to produce electricity.
From Figure 16.9 the total MSW (in the U.S.) in recent years has been about 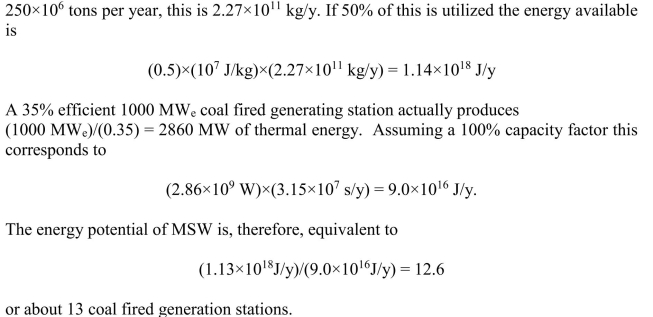

3
The average per capita rate of primary energy use in Canada is
approximately 13 kW. Spruce trees grow at an average rate of approximately 0.005 kg per
m2 of forest per day and have an average energy content of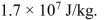 (a) If all energy in Canada were generated from spruce trees with the same average
(a) If all energy in Canada were generated from spruce trees with the same average
efficiency as present energy sources, what is the area of spruce forest needed to satisfy one
person's energy needs?
(b) What is the area of spruce forest needed to provide all of Canada's population with
energy? What fraction of the total land area does this represent?
approximately 13 kW. Spruce trees grow at an average rate of approximately 0.005 kg per
m2 of forest per day and have an average energy content of
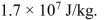 (a) If all energy in Canada were generated from spruce trees with the same average
(a) If all energy in Canada were generated from spruce trees with the same averageefficiency as present energy sources, what is the area of spruce forest needed to satisfy one
person's energy needs?
(b) What is the area of spruce forest needed to provide all of Canada's population with
energy? What fraction of the total land area does this represent?

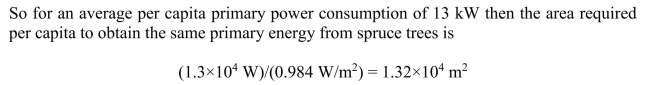
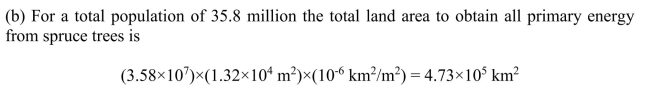
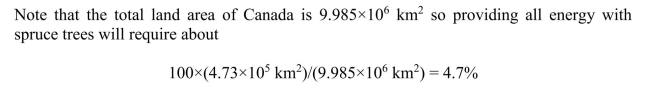 although much of the land area is in the arctic and not appropriate for tree growth.
although much of the land area is in the arctic and not appropriate for tree growth. 4
Photosynthesis is a very inefficient process for converting sunlight into
usable chemical energy. While the theoretical efficiency is 25%, the actual efficiency is
affected by the wavelength distribution of the light, the absorptance of the plant matter, and
various other factors and may typically be in the range of about 1%. Using the energy
content of wood as a guide and the average solar insolation of 168 W/m2 (Chapter 8), how
much land area would be needed to fulfill an average person's energy needs (in the United
States)? Assume that biomass energy content can be converted into end user energy with
the same efficiency as current energy sources (Chapter 2).
usable chemical energy. While the theoretical efficiency is 25%, the actual efficiency is
affected by the wavelength distribution of the light, the absorptance of the plant matter, and
various other factors and may typically be in the range of about 1%. Using the energy
content of wood as a guide and the average solar insolation of 168 W/m2 (Chapter 8), how
much land area would be needed to fulfill an average person's energy needs (in the United
States)? Assume that biomass energy content can be converted into end user energy with
the same efficiency as current energy sources (Chapter 2).

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the mass of CO2 (in kilograms) produced by the combustion of 1
kg of ethanol.
kg of ethanol.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
Following Example 16.3, estimate the necessary fuel tank volume and fuel
mass for a vehicle operating on E85 as compared to a gasoline vehicle in order to achieve
the same driving range.
mass for a vehicle operating on E85 as compared to a gasoline vehicle in order to achieve
the same driving range.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
The average wood content of forested areas in the United States is 250 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
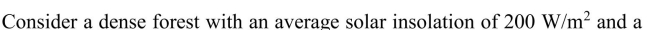


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
The light alcohols in Table 16.1 are referred to as monohydric alcohols and 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
A large maple tree collects sunlight over an area that is 8 m in diameter. The
average solar radiation (Chapter 8) is 168 W/m2. The tree grows for 8 months of the year
and is dormant for 4 months of the year. After 10 years, the mass of the tree has increased
by 540 kg. What is the efficiency of converting sunlight into chemical energy?
average solar radiation (Chapter 8) is 168 W/m2. The tree grows for 8 months of the year
and is dormant for 4 months of the year. After 10 years, the mass of the tree has increased
by 540 kg. What is the efficiency of converting sunlight into chemical energy?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Compare the relative amounts of CO2 produced per MJ of energy from the
combustion of methane and methanol.
combustion of methane and methanol.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
Two vehicles implement different approaches to using ethanol as a fuel.
One vehicle burns the ethanol in an internal combustion engine with an efficiency of 18%
to convert the chemical energy in the ethanol to mechanical energy supplied to the
vehicle's wheels. The second vehicle uses electricity to charge batteries to run electric
motors to drive the wheels. The electricity is produced by burning the ethanol in a heat
engine to drive a generator with a net efficiency of 38%. The batteries/electric motors are
89% efficient.
One vehicle burns the ethanol in an internal combustion engine with an efficiency of 18%
to convert the chemical energy in the ethanol to mechanical energy supplied to the
vehicle's wheels. The second vehicle uses electricity to charge batteries to run electric
motors to drive the wheels. The electricity is produced by burning the ethanol in a heat
engine to drive a generator with a net efficiency of 38%. The batteries/electric motors are
89% efficient.


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Two methods are used to generate 1 GWhe of electricity: (1) burning coal 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Estimate the total mass of fuelwood used in the United States per year.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
How many tonnes of wood per day would be needed for a wood-fired
generating station that produces 1000 MWe continuously (typical of a coal-fired facility) at
35% efficiency?
generating station that produces 1000 MWe continuously (typical of a coal-fired facility) at
35% efficiency?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
What fraction of arable land in the United States would need to be utilized
if biodiesel produced from sunflowers replaced all of the country's diesel requirements?
Repeat this calculation for biodiesel produced from algae.
if biodiesel produced from sunflowers replaced all of the country's diesel requirements?
Repeat this calculation for biodiesel produced from algae.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the mass of CO2 (in kilograms) per MJ of energy produced for the
hydrocarbons with n = 1 through 4 shown in Table 16.2.
hydrocarbons with n = 1 through 4 shown in Table 16.2.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
In North America each person produces approximately 2.0 kg of municipal
solid waste (MSW) per day. If this MSW is converted into electricity with an efficiency of
38%, what fraction of a person's average electric power use of 0.6 kW could be provided?
solid waste (MSW) per day. If this MSW is converted into electricity with an efficiency of
38%, what fraction of a person's average electric power use of 0.6 kW could be provided?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
A home owner uses 3000 L of oil annually for heat, using an 85% efficient
furnace. If the heating system is converted to a wood furnace operating at 70% efficiency,
what mass of wood is required annually to provide the same heating value?
furnace. If the heating system is converted to a wood furnace operating at 70% efficiency,
what mass of wood is required annually to provide the same heating value?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Methanol is a convenient fuel, because it is a liquid at standard temperature
and pressure. Methanol may be produced by first reacting carbon with water as in equation
(3.4) to produce hydrogen and carbon monoxide. The carbon monoxide may be reacted
with water to produce carbon dioxide and hydrogen as in equation (3.5). Methanol (along
with water) is produced by combining CO2 with hydrogen.
(a) Write the equations for this series of reactions and show that excess CO2 is produced.
(b) What is the ratio of the mass of the CO2 produced by methanol production to the mass
of the CO2 produced by subsequently burning the methanol?
and pressure. Methanol may be produced by first reacting carbon with water as in equation
(3.4) to produce hydrogen and carbon monoxide. The carbon monoxide may be reacted
with water to produce carbon dioxide and hydrogen as in equation (3.5). Methanol (along
with water) is produced by combining CO2 with hydrogen.
(a) Write the equations for this series of reactions and show that excess CO2 is produced.
(b) What is the ratio of the mass of the CO2 produced by methanol production to the mass
of the CO2 produced by subsequently burning the methanol?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck



