Deck 8: Acid-Base Balance
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 8: Acid-Base Balance
1
What is the pH of water?
A)6.3
B)7.0
C)7.5
D)8.0
A)6.3
B)7.0
C)7.5
D)8.0
B
2
The _____ regulate(s) the concentration of HCO₃-
A)pancreas
B)gallbladder
C)kidneys
D)liver
A)pancreas
B)gallbladder
C)kidneys
D)liver
C
3
Which of the following is considered a normal serum pH for humans?
A)5.75-6.35
B)6.95-7.25
C)7.35-7.45
D)7.6-7.9
A)5.75-6.35
B)6.95-7.25
C)7.35-7.45
D)7.6-7.9
C
4
When alkalosis occurs, the kidneys will respond by:
A)reducing the amount of HCO₃ reabsorbed.
B)increasing the amount of HCO₃ reabsorbed.
C)reducing the amount of hydrocarbonate.
D)excreting inadequate amounts of calcium.
A)reducing the amount of HCO₃ reabsorbed.
B)increasing the amount of HCO₃ reabsorbed.
C)reducing the amount of hydrocarbonate.
D)excreting inadequate amounts of calcium.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is an example of an inorganic nonvolatile acid?
A)phosphoric acid
B)lactic acid
C)hydroxybutyric acid
D)bicarbonate
A)phosphoric acid
B)lactic acid
C)hydroxybutyric acid
D)bicarbonate

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
Changes in the level of carbon dioxide are detected in the _____ of the brain
A)hypothalamus
B)medulla oblongata
C)cerebrospinal fluid
D)frontal lobe
A)hypothalamus
B)medulla oblongata
C)cerebrospinal fluid
D)frontal lobe

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the most predominant base in the body?
A)ammonia
B)bicarbonate
C)lithium hydroxide
D)sodium hydroxide
A)ammonia
B)bicarbonate
C)lithium hydroxide
D)sodium hydroxide

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
In regard to respiratory and renal control buffer systems, which of the following statements is true?
A)The kidneys work alone to respond to changes in pH.
B)The kidneys' response time is faster than the response time of the lungs.
C)The lungs work alone to respond to changes in pH.
D)The lungs' response time is faster than the response time of the kidneys.
A)The kidneys work alone to respond to changes in pH.
B)The kidneys' response time is faster than the response time of the lungs.
C)The lungs work alone to respond to changes in pH.
D)The lungs' response time is faster than the response time of the kidneys.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
The lungs regulate _____ levels, while the kidneys regulate _____ levels
A)carbon dioxide, bicarbonate
B)hydrogen ions, carbon dioxide
C)bicarbonate, hydrogen ions
D)oxygen, hydrogen ions
A)carbon dioxide, bicarbonate
B)hydrogen ions, carbon dioxide
C)bicarbonate, hydrogen ions
D)oxygen, hydrogen ions

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
An example of a volatile acid is
A)carbonic acid.
B)amino acid.
C)lactic acid.
D)phosphoric acid.
A)carbonic acid.
B)amino acid.
C)lactic acid.
D)phosphoric acid.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
PH is defined as the:
A)ratio of bases to acids.
B)ratio of volatile acids to nonvolatile acids.
C)ratio of acids to bases.
A)ratio of bases to acids.
B)ratio of volatile acids to nonvolatile acids.
C)ratio of acids to bases.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
Carbonic acid dissolves into _____ and _____
A)CO₂, water
B)hydrogen ions, bicarbonate
C)oxygen, carbon dioxide
D)hydrogen ions, oxygen
A)CO₂, water
B)hydrogen ions, bicarbonate
C)oxygen, carbon dioxide
D)hydrogen ions, oxygen

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
The most common base in humans:
A)is lactate.
B)comes mainly from ingestion of fruits.
C)comes mainly from ingestion of vegetables.
D)is bicarbonate.
A)is lactate.
B)comes mainly from ingestion of fruits.
C)comes mainly from ingestion of vegetables.
D)is bicarbonate.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
When pH imbalances occur, it may take as long as _____ hours for the renal regulation system to respond
A)12
B)24
C)36
D)48
A)12
B)24
C)36
D)48

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
The lungs work to get rid of an increased amount of acid by:
A)increasing the rate and depth of breathing.
B)increasing the rate and lowering the depth of breathing.
C)decreasing the rate and depth of breathing.
D)decreasing the rate and increasing the depth of breathing.
A)increasing the rate and depth of breathing.
B)increasing the rate and lowering the depth of breathing.
C)decreasing the rate and depth of breathing.
D)decreasing the rate and increasing the depth of breathing.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
Acids are defined as:
A)substances that donate hydrogen ions.
B)substances that have a ph level of 7.
C)substances that accept hydrogen ions.
D)substance that donate hydroxide ions.
A)substances that donate hydrogen ions.
B)substances that have a ph level of 7.
C)substances that accept hydrogen ions.
D)substance that donate hydroxide ions.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
The process that leads to an accumulation of acid or loss of base is known as:
A)acidemia.
B)acidosis.
C)alkalemia.
D)ascites.
A)acidemia.
B)acidosis.
C)alkalemia.
D)ascites.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
The most prominent chemical buffering system that is used to regulate pH is the relationship of HCO₃- to:
A)carbonic acid.
B)COs.
C)H+.
A)carbonic acid.
B)COs.
C)H+.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
Bases are defined as:
A)substances that donate hydrogen ions.
B)substances that have a ph level numerically lower than 7.
C)substances that can accept hydroxide ions.
D)substances that can accept hydrogen ions.
A)substances that donate hydrogen ions.
B)substances that have a ph level numerically lower than 7.
C)substances that can accept hydroxide ions.
D)substances that can accept hydrogen ions.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
If the pH of urine drops below _____, it can become harmful to the body
A)4.5
B)5.0
C)5.2
D)5.5
A)4.5
B)5.0
C)5.2
D)5.5

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
Case Study Multiple Choice
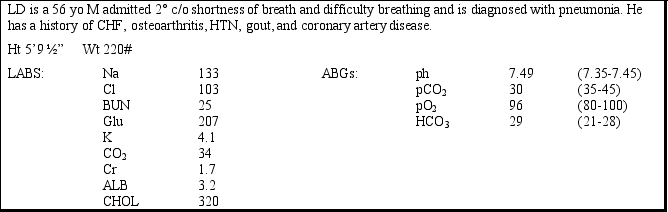
Which of the following is an indication that LD has an acid-base disorder?
A)low Na
B)high pH
C)low albumin
D)his diagnosis

Which of the following is an indication that LD has an acid-base disorder?
A)low Na
B)high pH
C)low albumin
D)his diagnosis

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
All of the following can result in metabolic acidosis except:
A)starvation.
B)ingestion of formaldehyde.
C)hyperventilation.
D)overdosing of aspirin.
A)starvation.
B)ingestion of formaldehyde.
C)hyperventilation.
D)overdosing of aspirin.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
23
Your patient has emphysema and is now infected with bronchial pneumonia Due to hyperventilation, he is at risk for:
A)respiratory alkalosis.
B)respiratory acidosis.
C)metabolic acidosis.
D)metabolic alkalosis.
A)respiratory alkalosis.
B)respiratory acidosis.
C)metabolic acidosis.
D)metabolic alkalosis.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
Case Study Multiple Choice

What acid-base disorder does LD have?
A)metabolic alkalosis
B)metabolic acidosis
C)respiratory alkalosis
D)respiratory acidosis

What acid-base disorder does LD have?
A)metabolic alkalosis
B)metabolic acidosis
C)respiratory alkalosis
D)respiratory acidosis

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
All of the following except _____ can disturb fluid balance and can lead to metabolic alkalosis
A)prolonged vomiting
B)diuretics
C)statins
D)nasogastric suction
A)prolonged vomiting
B)diuretics
C)statins
D)nasogastric suction

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
All of the following except for _____ can lead to respiratory acidosis
A)pneumonia
B)hypertension
C)acute pulmonary edema
D)pneumothorax
A)pneumonia
B)hypertension
C)acute pulmonary edema
D)pneumothorax

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
Case Study Multiple Choice

The above would result in:
A)a lower pH.
B)an increased pH.
C)an increased pCO₃.
D)an increased pCO₂.

The above would result in:
A)a lower pH.
B)an increased pH.
C)an increased pCO₃.
D)an increased pCO₂.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following medications are involved in the etiology of respiratory acidosis?
A)blood thinners
B)statins
C)opiates
D)diuretics
A)blood thinners
B)statins
C)opiates
D)diuretics

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
All of the following are symptoms of respiratory alkalosis except:
A)anxiety.
B)increased appetite.
C)mental confusion.
D)seizures.
A)anxiety.
B)increased appetite.
C)mental confusion.
D)seizures.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
Metabolic alkalosis is a result of which of the following?
A)low amount of base
B)excessive amount of base
C)low amount of acid
D)excessive amount of acid
A)low amount of base
B)excessive amount of base
C)low amount of acid
D)excessive amount of acid

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
Common laboratory measurements used to assess acid-base balance include all of the following except for:
A)carbon dioxide.
B)anion gap.
C)ammonia.
D)bicarbonate.
A)carbon dioxide.
B)anion gap.
C)ammonia.
D)bicarbonate.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
Case Study Multiple Choice

If this compensatory disorder were to develop, what would be a likely scenario?
A)the lungs would slow down respirations
B)the lungs would increase respirations
C)the kidneys would reduce their secretion of H+
D)the kidneys would increase their secretion of H+

If this compensatory disorder were to develop, what would be a likely scenario?
A)the lungs would slow down respirations
B)the lungs would increase respirations
C)the kidneys would reduce their secretion of H+
D)the kidneys would increase their secretion of H+

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
Respiratory alkalosis is generally a result of conditions that cause which of the following?
A)lethargy
B)restlessness
C)muscle twitching
D)hyperventilation
A)lethargy
B)restlessness
C)muscle twitching
D)hyperventilation

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
Stored blood contains _____; therefore, large blood transfusions could potentially lead to metabolic alkalosis
A)bicabornate
B)calcium
C)citrate
D)malate
A)bicabornate
B)calcium
C)citrate
D)malate

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
Case Study Multiple Choice

What factor is contributing to his acid-base disorder?
A)gout
B)shortness of breath
C)coronary artery disease
D)HTN

What factor is contributing to his acid-base disorder?
A)gout
B)shortness of breath
C)coronary artery disease
D)HTN

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
Case Study Multiple Choice

What compensatory disorder could he develop?
A)metabolic alkalosis
B)metabolic acidosis
C)respiratory alkalosis
D)respiratory acidosis

What compensatory disorder could he develop?
A)metabolic alkalosis
B)metabolic acidosis
C)respiratory alkalosis
D)respiratory acidosis

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
Match between columns

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck



