Deck 3: Elementary Polymer Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/23
Play
Full screen (f)
Deck 3: Elementary Polymer Chemistry
1
Which type of chemical bonding is most important in plastics?
A) metallic
B) ionic
C) hydrogen
D) covalent
A) metallic
B) ionic
C) hydrogen
D) covalent
D
2
As the number of carbons in the backbone of a hydrocarbon increases, the molecules get longer and longer and the substance is most likely to be a ____.
A) solid
B) gas
C) liquid
D) vapor
A) solid
B) gas
C) liquid
D) vapor
A
3
Unsaturated molecules contain some ____ bonds.
A) hydrogen
B) metallic
C) double
D) ionic
A) hydrogen
B) metallic
C) double
D) ionic
C
4
The process of ____ usually involves applying controlled heating of parts of plastics.
A) orientation
B) crystallization
C) uncoiling
D) annealing
A) orientation
B) crystallization
C) uncoiling
D) annealing

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
5
Plastics always contain the element ____.
A) carbon
B) silicon
C) oxygen
D) nitrogen
A) carbon
B) silicon
C) oxygen
D) nitrogen

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
6
This configuration is of the hydrocarbon, ____.
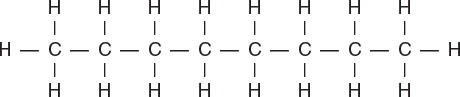
A) methane
B) pentane
C) octane
D) hexane

A) methane
B) pentane
C) octane
D) hexane

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
7
Rigid thermosets often find applications in ____ environments.
A) high-heat
B) moist
C) frigid
D) extremely dry
A) high-heat
B) moist
C) frigid
D) extremely dry

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
8
In an amorphous molecular structure of plastic, the appearance of the plastic will always be ____.
A) reflective
B) opaque
C) transparent
D) translucent
A) reflective
B) opaque
C) transparent
D) translucent

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
9
Examples of a flexible thermosets is/are ____.
A) hydrofoils
B) automobile hoods
C) safety glass
D) foams for car seats
A) hydrofoils
B) automobile hoods
C) safety glass
D) foams for car seats

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
10
The joining together of many mers is known as ____.
A) a homopolymer
B) paraffin
C) ethane
D) polymerization
A) a homopolymer
B) paraffin
C) ethane
D) polymerization

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
11
An example of a plastics product that shows orientation in its stretch differences, stretching easily in the crosswise direction, but not so readily lengthwise is ____.
A) PVC
B) teflon tape
C) duct tape
D) Shrinky-Dinks®
A) PVC
B) teflon tape
C) duct tape
D) Shrinky-Dinks®

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
12
An example of a plastics product that shows a biaxial orientation in that it will stretch in two directions if heated is ____.
A) PVC
B) teflon tape
C) a pop bottle
D) thin film labels
A) PVC
B) teflon tape
C) a pop bottle
D) thin film labels

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
13
Plastics that consist of disconnected hydrocarbon chains are called ____.
A) biaxial plastics
B) cross links
C) thermosets
D) thermoplastics
A) biaxial plastics
B) cross links
C) thermosets
D) thermoplastics

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
14
Which combination of atoms represents a permanent dipole?
A) a carbon-fluorine single bond
B) a carbon-fluorine double bond
C) a nitrogen-hydrogen single bond
D) a carbon-OH single bond
A) a carbon-fluorine single bond
B) a carbon-fluorine double bond
C) a nitrogen-hydrogen single bond
D) a carbon-OH single bond

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
15
Which saturated hydrocarbons molecule contains three carbon atoms?
A) butane
B) propane
C) methane
D) octane
A) butane
B) propane
C) methane
D) octane

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
16
When two or more atoms combine, the resulting structure is known as a(n) ____.
A) isomer
B) molecule
C) isotope
D) polymer
A) isomer
B) molecule
C) isotope
D) polymer

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
17
When hydrocarbon molecules have only single covalent bonds, they are considered ____.
A) saturated
B) hydrolyzed
C) monounsaturated
D) polyunsaturated
A) saturated
B) hydrolyzed
C) monounsaturated
D) polyunsaturated

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
18
In PVC, a ____ atom replaces one hydrogen atom per mer.
A) sodium
B) sulfur
C) bromine
D) chlorine
A) sodium
B) sulfur
C) bromine
D) chlorine

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
19
An intermolecular interaction in which molecules or portions of molecules exhibit polarity is known as a(n) ____.
A) Van der Waals force
B) dipole interaction
C) hydrogen bond
D) ionic bond
A) Van der Waals force
B) dipole interaction
C) hydrogen bond
D) ionic bond

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
20
When crystalline plastics are melted, the crystalline regions unfold, and the entire structure becomes ____.
A) annealed
B) polarized
C) amorphous
D) uniaxial
A) annealed
B) polarized
C) amorphous
D) uniaxial

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
21
A benzene molecule has the shape of a ____.
A) straight chain
B) ring
C) kinked chain
D) crystal
A) straight chain
B) ring
C) kinked chain
D) crystal

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
22
How many carbon atoms does decane have?
A) 2
B) 5
C) 8
D) 10
A) 2
B) 5
C) 8
D) 10

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
23
Which molecule is a solid?
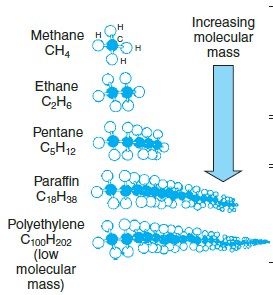
A) polethylene
B) methane
C) ethane
D) pentane

A) polethylene
B) methane
C) ethane
D) pentane

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck



