Deck 2: Atoms and Molecules the Chemical Basis of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Question
Match between columns
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/69
Play
Full screen (f)
Deck 2: Atoms and Molecules the Chemical Basis of Life
1
Figure 2-1 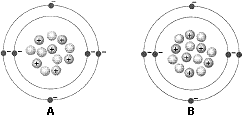 What is the atomic mass of the atom identified as A in the accompanying figure?
What is the atomic mass of the atom identified as A in the accompanying figure?
A) 2 amu
B) 6 amu
C) 8 amu
D) 12 amu
E) 18 amu
 What is the atomic mass of the atom identified as A in the accompanying figure?
What is the atomic mass of the atom identified as A in the accompanying figure?A) 2 amu
B) 6 amu
C) 8 amu
D) 12 amu
E) 18 amu
D
2
How can we identify a particular element?
A) By its number of protons
B) By its number of electrons
C) By its number of neutrons
D) By its shape of valence shells
E) By its amount of energy levels
A) By its number of protons
B) By its number of electrons
C) By its number of neutrons
D) By its shape of valence shells
E) By its amount of energy levels
A
3
The representation H − O − H is known as a(n):
A) structural formula.
B) simplest formula.
C) molecular formula.
D) Lewis structure.
E) orbital diagram.
A) structural formula.
B) simplest formula.
C) molecular formula.
D) Lewis structure.
E) orbital diagram.
A
4
The location and/or metabolism of a substance such as a hormone or drug can be followed in the body by labeling the substance with a(n):
A) acid
B) isotope
C) element
D) radioisotope
E) DNA molecule
A) acid
B) isotope
C) element
D) radioisotope
E) DNA molecule

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
5
Which best statement describes an element?
A) A substance that cannot burn.
B) A substance that is soluble in both acid and base
C) A substance that is held together by covalent bonds
D) A substance that is composed of more than one kind of atom
E) A substance that cannot be broken into simpler substances by chemical reactions
A) A substance that cannot burn.
B) A substance that is soluble in both acid and base
C) A substance that is held together by covalent bonds
D) A substance that is composed of more than one kind of atom
E) A substance that cannot be broken into simpler substances by chemical reactions

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
6
Figure 2-1 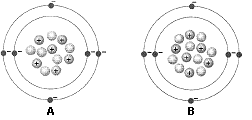 What is the difference between the two atoms in the accompanying figure?
What is the difference between the two atoms in the accompanying figure?
A) Their electrical charge
B) The number of electrons
C) The number of protons
D) The number of neutrons
E) The number of valence shells
 What is the difference between the two atoms in the accompanying figure?
What is the difference between the two atoms in the accompanying figure?A) Their electrical charge
B) The number of electrons
C) The number of protons
D) The number of neutrons
E) The number of valence shells

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
7
In a water molecule, because oxygen is more electronegative than hydrogen, the shared electrons are more commonly found around the ____ nucleus than the ____ nucleus.
A) oxygen; hydrogen
B) hydrogen; oxygen
C) hydrogen; other hydrogen
D) oxygen; nitrogen
E) nitrogen; oxygen
A) oxygen; hydrogen
B) hydrogen; oxygen
C) hydrogen; other hydrogen
D) oxygen; nitrogen
E) nitrogen; oxygen

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
8
What type of bond is formed if one atom is more electronegative than the other atom?
A) covalent
B) hydrogen
C) polar covalent
D) van der Waals
E) nonpolar covalent
A) covalent
B) hydrogen
C) polar covalent
D) van der Waals
E) nonpolar covalent

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
9
Which substance correctly identifies a reactant in the following chemical equation? CO2 + H2O ↔ H2CO3
A) water
B) carbon
C) oxygen
D) hydrogen
E) carbonic acid
A) water
B) carbon
C) oxygen
D) hydrogen
E) carbonic acid

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
10
The chemical behavior of an atom is determined most directly by the:
A) atomic number.
B) atomic weight.
C) number of neutrons.
D) number of energy levels.
E) number of valence electrons.
A) atomic number.
B) atomic weight.
C) number of neutrons.
D) number of energy levels.
E) number of valence electrons.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
11
The molecular mass of C6H12O6 is 180 amu. How much mass does 0.25 moles of this substance contain?
A) 1.8 g
B) 45 g
C) 180 g
D) 45 daltons
E) 180 daltons
A) 1.8 g
B) 45 g
C) 180 g
D) 45 daltons
E) 180 daltons

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
12
How many molecules are present in one mole of C6H12O6?
A) 1.7 × 10 − 10 molecules
B) 1.3 × 1010 molecules
C) 24 molecules
D) 1.7 × 1022 molecules
E) 6.02 × 1023 molecules
A) 1.7 × 10 − 10 molecules
B) 1.3 × 1010 molecules
C) 24 molecules
D) 1.7 × 1022 molecules
E) 6.02 × 1023 molecules

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
13
What differentiates an organic compound from an inorganic compound?
A) An organic compound lacks isotopes.
B) An organic compound contains carbon.
C) An organic compound lacks valence electrons.
D) An organic compound is basic rather than acidic.
E) An organic compound contains two or more atoms.
A) An organic compound lacks isotopes.
B) An organic compound contains carbon.
C) An organic compound lacks valence electrons.
D) An organic compound is basic rather than acidic.
E) An organic compound contains two or more atoms.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
14
Which description best illustrates the product of a chemical reaction?
A) It is joined by an ionic bond only.
B) It is always in equilibrium with the reactants.
C) It is the substance that initiates the reaction.
D) It is generally written on the left side of the equation.
E) It is generally written on the right side and is the substance generated by the reaction.
A) It is joined by an ionic bond only.
B) It is always in equilibrium with the reactants.
C) It is the substance that initiates the reaction.
D) It is generally written on the left side of the equation.
E) It is generally written on the right side and is the substance generated by the reaction.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
15
Figure 2-1 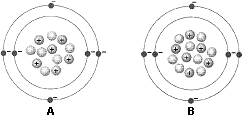 What does the accompanying figure represent?
What does the accompanying figure represent?
A) an acid and a base
B) two different ions
C) a cation and an anion
D) two different elements
E) two isotopes of the same element
 What does the accompanying figure represent?
What does the accompanying figure represent?A) an acid and a base
B) two different ions
C) a cation and an anion
D) two different elements
E) two isotopes of the same element

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
16
A chlorine atom has 17 protons and 18 neutrons. What is its atomic mass?
A) 1 amu
B) 17 amu
C) 18 amu
D) 35 amu
E) 306 amu
A) 1 amu
B) 17 amu
C) 18 amu
D) 35 amu
E) 306 amu

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
17
Nitrogen has five electrons in its valence shell. How many electrons does it need to gain to complete its valence shell?
A) one
B) two
C) three
D) seven
E) eight
A) one
B) two
C) three
D) seven
E) eight

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
18
What is the difference between a stable isotope and a radioisotope?
A) A stable isotope emits light.
B) A radioisotope emits radiation.
C) A stable isotope emits radiation.
D) A stable isotope absorbs radiation.
E) A radioisotope has an unequal number of protons and electrons.
A) A stable isotope emits light.
B) A radioisotope emits radiation.
C) A stable isotope emits radiation.
D) A stable isotope absorbs radiation.
E) A radioisotope has an unequal number of protons and electrons.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
19
An atom X contains 14 protons, 13 electrons, and 12 neutrons, and atom Y contains 14 protons, 14 electrons, and 12 neutrons. What can you conclude?
A) Y is an ion but X is not.
B) X and Y are both ions.
C) X and Y both have filled valence shells.
D) X and Y are isotopes of the same element.
E) X and Y are atoms of the same element.
A) Y is an ion but X is not.
B) X and Y are both ions.
C) X and Y both have filled valence shells.
D) X and Y are isotopes of the same element.
E) X and Y are atoms of the same element.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
20
Isotopes differ from each other with respect to the number of:
A) protons only.
B) electrons only.
C) neutrons only.
D) both protons and electrons.
E) both neutrons and protons.
A) protons only.
B) electrons only.
C) neutrons only.
D) both protons and electrons.
E) both neutrons and protons.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
21
It takes one calorie of heat to raise the temperature of one gram of water one degree Celsius at sea level. This is referred to as the ____ of water.
A) specific heat
B) heat of fusion
C) homeostasis
D) vaporization
E) heat of transformation
A) specific heat
B) heat of fusion
C) homeostasis
D) vaporization
E) heat of transformation

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
22
What is the difference between an electrically neutral atom and an ion?
A) An ion has an unequal number of protons and electrons, while an atom has an equal number.
B) An ion has an equal number of protons and electrons, while an atom has an unequal number.
C) An atom has an unequal number of neutrons and protons, while an ion has an equal number.
D) An atom has its electrons in orbitals, while an ion has its electrons in its nucleus.
E) An atom must have an equal number of neutrons and electrons, while an ion does not.
A) An ion has an unequal number of protons and electrons, while an atom has an equal number.
B) An ion has an equal number of protons and electrons, while an atom has an unequal number.
C) An atom has an unequal number of neutrons and protons, while an ion has an equal number.
D) An atom has its electrons in orbitals, while an ion has its electrons in its nucleus.
E) An atom must have an equal number of neutrons and electrons, while an ion does not.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
23
Which component is the oxidizing agent in the following chemical reaction? 4 Fe + 3 O2 → 2 Fe2O3
A) rust
B) iron
C) water
D) oxygen
E) hydrogen
A) rust
B) iron
C) water
D) oxygen
E) hydrogen

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
24
Which atom would most likely be involved in an ionic bond?
A) hydrogen
B) oxygen
C) sodium
D) nitrogen
E) helium
A) hydrogen
B) oxygen
C) sodium
D) nitrogen
E) helium

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
25
The covalent bond between a hydrogen atom and the oxygen atom in water is formed when:
A) hydrogen gains an electron from oxygen.
B) hydrogen and oxygen share an electron pair.
C) hydrogen and oxygen both lose electrons from their outer shells.
D) hydrogen and oxygen both gain electrons in their outer shells.
E) hydrogen gains an electron from oxygen.
A) hydrogen gains an electron from oxygen.
B) hydrogen and oxygen share an electron pair.
C) hydrogen and oxygen both lose electrons from their outer shells.
D) hydrogen and oxygen both gain electrons in their outer shells.
E) hydrogen gains an electron from oxygen.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
26
Why can table salt (NaCl) dissolve easily in water?
A) Water can add electrons to the sodium ion.
B) Water can form covalent linkages with salt molecules.
C) Water can remove electrons from the chloride ion, which causes the latter to dissociate from the sodium and dissolve.
D) Water is polar and salt is nonpolar. Nonpolar compounds are more soluble in polar solvents because they are able to form strong covalent bonds that result in a breaking up of the molecule being dissolved.
E) The partial positive charge of the hydrogens in the water molecule can associate with the negative charge of the chloride ion, and the partial negative charge of the oxygen of the water molecule can associate with the positive charge of the sodium atom.
A) Water can add electrons to the sodium ion.
B) Water can form covalent linkages with salt molecules.
C) Water can remove electrons from the chloride ion, which causes the latter to dissociate from the sodium and dissolve.
D) Water is polar and salt is nonpolar. Nonpolar compounds are more soluble in polar solvents because they are able to form strong covalent bonds that result in a breaking up of the molecule being dissolved.
E) The partial positive charge of the hydrogens in the water molecule can associate with the negative charge of the chloride ion, and the partial negative charge of the oxygen of the water molecule can associate with the positive charge of the sodium atom.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the aquatic insects pictured in the accompanying figure. Which characteristic of water molecules directly contributes to their remarkable "water walking" success? 
A) ionic bonds
B) capillary action
C) hydrogen bonds
D) adhesive forces
E) nonpolar covalent bonds

A) ionic bonds
B) capillary action
C) hydrogen bonds
D) adhesive forces
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following would most likely form electrolytes in water?
A) glucose
B) ethanol
C) an organic compound
D) an inorganic compound
E) a nonionic compound
A) glucose
B) ethanol
C) an organic compound
D) an inorganic compound
E) a nonionic compound

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
29
A base is defined as a(n) _____ acceptor.
A) neutron
B) electron
C) proton
D) anion
E) cation
A) neutron
B) electron
C) proton
D) anion
E) cation

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
30
The cohesiveness between water molecules is due largely to:
A) ionic bonds.
B) hydrogen bonds.
C) polar covalent bonds.
D) nonpolar covalent bonds.
E) hydrophobic interactions.
A) ionic bonds.
B) hydrogen bonds.
C) polar covalent bonds.
D) nonpolar covalent bonds.
E) hydrophobic interactions.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
31
Which statement about van der Waal interactions is false ?
A) They are very strong.
B) They are attractive forces.
C) They operate over very short distances.
D) They form between nonpolar molecules.
E) They involve transient regions of positive and negative charges.
A) They are very strong.
B) They are attractive forces.
C) They operate over very short distances.
D) They form between nonpolar molecules.
E) They involve transient regions of positive and negative charges.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
32
A stalk of celery is placed in a solution of blue colored dye. After one hour, the leaves have blue fluid in their veins. Which property of water is being demonstrated?
A) surface tension
B) high specific heat
C) adhesion and cohesion
D) evaporation and cooling
E) lower density as a solid than as a liquid
A) surface tension
B) high specific heat
C) adhesion and cohesion
D) evaporation and cooling
E) lower density as a solid than as a liquid

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
33
Figure 2-2  The type of bond illustrated in the accompanying diagram is a(n):
The type of bond illustrated in the accompanying diagram is a(n):
A) ionic bond.
B) polar bond.
C) hydrogen bond.
D) single covalent bond.
E) double covalent bond.
 The type of bond illustrated in the accompanying diagram is a(n):
The type of bond illustrated in the accompanying diagram is a(n):A) ionic bond.
B) polar bond.
C) hydrogen bond.
D) single covalent bond.
E) double covalent bond.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
34
When does an atom becomes a cation?
A) It emits radiation.
B) It shares electrons.
C) It loses one or more electron.
D) It gains one or more electron.
E) One or more of its electrons changes energy levels.
A) It emits radiation.
B) It shares electrons.
C) It loses one or more electron.
D) It gains one or more electron.
E) One or more of its electrons changes energy levels.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
35
Which event illustrates evaporative cooling?
A) a tea kettle whistling
B) sweat evaporating from the skin
C) ice cubes floating in a glass of water
D) a fish not freezing in a pond covered with ice
E) salt dissolving in a pot of water full of potatoes
A) a tea kettle whistling
B) sweat evaporating from the skin
C) ice cubes floating in a glass of water
D) a fish not freezing in a pond covered with ice
E) salt dissolving in a pot of water full of potatoes

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
36
Sugar dissolves readily in water because it is a(n) ____ substance.
A) adhesive
B) cohesive
C) hydrophilic
D) hydrophobic
E) evaporative
A) adhesive
B) cohesive
C) hydrophilic
D) hydrophobic
E) evaporative

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
37
What is the OH − concentration of a solution having a pH of 2?
A) 1 × 10 − 12
B) 1 × 10 − 10
C) 1 × 10 − 7
D) 1 × 10 − 2
E) 1 × 10 − 1
A) 1 × 10 − 12
B) 1 × 10 − 10
C) 1 × 10 − 7
D) 1 × 10 − 2
E) 1 × 10 − 1

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
38
Which component becomes oxidized in the following chemical reaction? 4 Fe + 3 O2 → 2 Fe2O3
A) rust
B) iron
C) water
D) oxygen
E) hydrogen
A) rust
B) iron
C) water
D) oxygen
E) hydrogen

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
39
At what temperature is water most dense?
A) 0 degrees Celsius
B) 1 degree Celsius
C) 4 degrees Celsius
D) 10 degrees Celsius
E) 100 degrees Celsius
A) 0 degrees Celsius
B) 1 degree Celsius
C) 4 degrees Celsius
D) 10 degrees Celsius
E) 100 degrees Celsius

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
40
As water boils and turns to steam, what happens to the hydrogen bonds?
A) The hydrogen bonds break.
B) The hydrogen bonds become stronger.
C) The hydrogen bonds generate additional bonds.
D) The hydrogen bonds form a crystalline lattice structure.
E) The hydrogen bonds continually break and join together again.
A) The hydrogen bonds break.
B) The hydrogen bonds become stronger.
C) The hydrogen bonds generate additional bonds.
D) The hydrogen bonds form a crystalline lattice structure.
E) The hydrogen bonds continually break and join together again.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
41
List the four elements that account for over 90% of the mass of living organisms and identify an important biological function of each element.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
42
What is the purpose of a buffer?
A) To convert an acid to a base
B) To minimize the change in pH
C) To measure the acidity of a solution
D) To form a stable bond between atoms
E) To maintain a constant internal temperature
A) To convert an acid to a base
B) To minimize the change in pH
C) To measure the acidity of a solution
D) To form a stable bond between atoms
E) To maintain a constant internal temperature

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
43
The valence shell of hydrogen or helium is unstable when it contains two electrons.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
44
Explain how the number of valence electrons is related to the chemical properties of an atom. Use two specific examples in your explanation.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
45
Diagram and carefully label two water molecules using a ball-and-stick model. Then use this diagram to demonstrate how hydrogen bonds form between them.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
46
An atom having a filled valence shell is stable and unreactive .
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
47
A(n) inorganic compound is one that contains carbon.
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
48
A(n) chemical formula shows the types and numbers of atoms in a molecule and their arrangement.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
49
A salt is a compound in which the hydrogen ion of ____ is replaced by some other cation.
A) water
B) a base
C) an acid
D) an anion
E) a hydroxide ion
A) water
B) a base
C) an acid
D) an anion
E) a hydroxide ion

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
50
Which substance is an example of a very strong acid?
A) milk
B) coffee
C) bleach
D) seawater
E) lemon juice
A) milk
B) coffee
C) bleach
D) seawater
E) lemon juice

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
51
The atomic mass determines the type of element.
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
52
When a small amount of hydrochloric acid (HCl) is added to a solution of Na2HPO4, the pH of the solution does not change markedly. The pH also does not change drastically when a small amount of sodium hydroxide (NaOH) is added to this same solution. Based on these observations, the compound Na2HPO4 is:
A) acting as a buffer.
B) acting as a solvent.
C) able to donate hydrogen atoms to HCl.
D) able to remove hydrogen ions from the OH − of NaOH.
E) an enzyme facilitating the reaction between HCl and NaOH.
A) acting as a buffer.
B) acting as a solvent.
C) able to donate hydrogen atoms to HCl.
D) able to remove hydrogen ions from the OH − of NaOH.
E) an enzyme facilitating the reaction between HCl and NaOH.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
53
An example of a(n) anion is K+.
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
54
Oxidation occurs when an atom gains one or more electrons.
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
55
When atoms react to form an ionic bond, electrons are shared between those atoms.
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
56
Compare and contrast the formation, properties, and characteristics of covalent and ionic bonds.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
57
What is the approximate pH of ammonia?
A) 2
B) 4
C) 7
D) 8
E) 11
A) 2
B) 4
C) 7
D) 8
E) 11

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
58
A liquid with a pH of 7 is considered a(n) ____ solution.
A) basic
B) acidic
C) neutral
D) hydrophilic
E) hydrochloric
A) basic
B) acidic
C) neutral
D) hydrophilic
E) hydrochloric

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
59
If the hydrogen ion concentration of ammonia is 0.00000000001 mol/L, then what is its pH value?
A) 1
B) 10
C) -10
D) 11
E) -11
A) 1
B) 10
C) -10
D) 11
E) -11

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
60
The tetrahedron shape of a methane molecule is the result of orbital hybridization .
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
61
Match between columns

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
62
Specific heat refers to the amount of energy required to change 1 gram of a substance from the liquid phase to the vapor phase.
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
63
As a researcher, you are charged with determining the side effects of a new drug. From previous observations, you suspect that this drug reduces the rate of DNA production (replication) within skin cells of patients using the drug. With the following materials, design an experiment that would answer your questions about the effect of the drug on DNA production. You know that: DNA contains phosphate groups. You have: radioactive isotopes of phosphate (32P), skin cell cultures from various patients, the drug in question, and a device that measures radioactivity.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
64
Match between columns

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
65
Explain the role of carbon dioxide in maintaining blood pH levels.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
66
Water is most dense at 4 C .
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
67
A solution having a pH of 8 is slightly acidic .
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
68
The hydrogen bonds of water play an important role in the ability of animals to regulate their body temperature. Explain how this occurs.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
69
A substance that is resistant to changes in pH is called a(n) buffer .
__________________
__________________

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck



