Deck 3: The Chemistry of Life Organic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Premises:
contains purines and pyrimidines
contains purines and pyrimidines
contains purines and pyrimidines
contains purines and pyrimidines
cellulose
cellulose
cellulose
cellulose
most are nonpolar
most are nonpolar
most are nonpolar
most are nonpolar
consist of monomers having 20 different types
consist of monomers having 20 different types
consist of monomers having 20 different types
consist of monomers having 20 different types
Responses:
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 3: The Chemistry of Life Organic Compounds
1
What carbohydrate energy storage molecule is found in animal liver and muscle cells?
A) starch
B) cellulose
C) glycogen
D) a fatty acid
E) cholesterol
A) starch
B) cellulose
C) glycogen
D) a fatty acid
E) cholesterol
C
2
Unlike lipids, hydrophilic functional groups typically contain ____ atoms, which make them more soluble in water.
A) carbon
B) oxygen
C) hydrogen
D) nitrogen
E) phosphate
A) carbon
B) oxygen
C) hydrogen
D) nitrogen
E) phosphate
B
3
Which is an example of a disaccharide?
A) ribose
B) glucose
C) maltose
D) fructose
E) tricylglycerol
A) ribose
B) glucose
C) maltose
D) fructose
E) tricylglycerol
C
4
What group of molecules is represented in this structure? 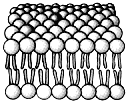
A) structural proteins
B) polysaccharides
C) triacylglycerols
D) phospholipids
E) polypeptides

A) structural proteins
B) polysaccharides
C) triacylglycerols
D) phospholipids
E) polypeptides

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
What substance is removed during a condensation reaction?
A) water
B) a dimer
C) a polymer
D) a hydrocarbon
E) a carboxyl group
A) water
B) a dimer
C) a polymer
D) a hydrocarbon
E) a carboxyl group

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
Why are hydrocarbons considered hydrophobic?
A) Hydrocarbons exist as isomers.
B) Hydrocarbons contain oxygen atoms.
C) The covalent bonds between carbon atoms are polar.
D) The covalent bonds between hydrogen and carbon are nonpolar.
E) The hydrogen bonds between hydrogen and carbon are nonpolar.
A) Hydrocarbons exist as isomers.
B) Hydrocarbons contain oxygen atoms.
C) The covalent bonds between carbon atoms are polar.
D) The covalent bonds between hydrogen and carbon are nonpolar.
E) The hydrogen bonds between hydrogen and carbon are nonpolar.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Amyloplasts are organelles that store:
A) fat.
B) starch.
C) protein.
D) lipids.
E) DNA.
A) fat.
B) starch.
C) protein.
D) lipids.
E) DNA.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
An amino group is weakly basic and includes a(n) ____ atom covalently bonded to two hydrogen atoms.
A) sulfur
B) carbon
C) oxygen
D) nitrogen
E) phosphate
A) sulfur
B) carbon
C) oxygen
D) nitrogen
E) phosphate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Which description illustrates an amphipathic molecule?
A) A phospholipid with two polar ends
B) A phospholipid with two hydrophobic ends
C) A phospholipid with only one hydrophilic end
D) A phospholipid with only one hydrophobic end
E) A phospholipid with both a hydrophobic end and a hydrophilic end
A) A phospholipid with two polar ends
B) A phospholipid with two hydrophobic ends
C) A phospholipid with only one hydrophilic end
D) A phospholipid with only one hydrophobic end
E) A phospholipid with both a hydrophobic end and a hydrophilic end

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
A molecule of a saturated triacylglycerol contains:
A) the maximum number of double bonds between carbons in the fatty acid chains.
B) the maximum number of triple bonds between carbons in the fatty acid chains.
C) the maximum number of hydrogen atoms in the fatty acid chains.
D) fatty acid chains with both amino and carboxyl groups.
E) alternating single and double bonds between carbons in the fatty acid chains.
A) the maximum number of double bonds between carbons in the fatty acid chains.
B) the maximum number of triple bonds between carbons in the fatty acid chains.
C) the maximum number of hydrogen atoms in the fatty acid chains.
D) fatty acid chains with both amino and carboxyl groups.
E) alternating single and double bonds between carbons in the fatty acid chains.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Which carbohydrate is the most structurally complex?
A) polymer
B) monomer
C) phospholipid
D) polysaccharide
E) monosaccharide
A) polymer
B) monomer
C) phospholipid
D) polysaccharide
E) monosaccharide

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Figure 3-1 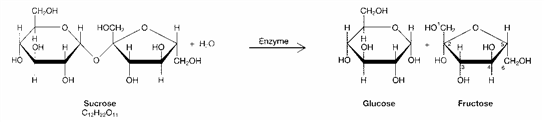 What chemical process is represented in the accompanying figure?
What chemical process is represented in the accompanying figure?
A) hydrolysis
B) denaturation
C) condensation
D) protein synthesis
E) dehydration synthesis
 What chemical process is represented in the accompanying figure?
What chemical process is represented in the accompanying figure?A) hydrolysis
B) denaturation
C) condensation
D) protein synthesis
E) dehydration synthesis

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Figure 3-1 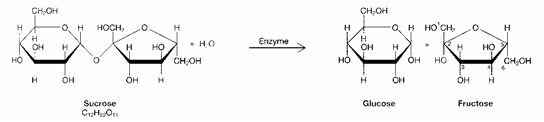 The products of the process in the accompanying figure are:
The products of the process in the accompanying figure are:
A) enzymes.
B) amino acids.
C) monosaccharides.
D) molecules of glycerol.
E) representative of a glycoside linkage.
 The products of the process in the accompanying figure are:
The products of the process in the accompanying figure are:A) enzymes.
B) amino acids.
C) monosaccharides.
D) molecules of glycerol.
E) representative of a glycoside linkage.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
How many carbon sugars are there in a pentose sugar?
A) two
B) three
C) five
D) six
E) eight
A) two
B) three
C) five
D) six
E) eight

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
If you partially hydrogenate oleic acid, the resulting molecule most likely would:
A) lose a carbon atom.
B) lose a carboxyl group.
C) become soluble in water.
D) contain more double bonds.
E) have a double bond changed from cis to trans.
A) lose a carbon atom.
B) lose a carboxyl group.
C) become soluble in water.
D) contain more double bonds.
E) have a double bond changed from cis to trans.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
When we know what kinds of ____ are present in an organic compound, we can predict its chemical behavior.
A) proteins
B) enzymes
C) triacylglycerols
D) macomolecules
E) functional groups
A) proteins
B) enzymes
C) triacylglycerols
D) macomolecules
E) functional groups

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Which is a property of unsaturated fats?
A) They are more common in animals.
B) They are generally liquid at room temperature.
C) They have no double bonds in the carbon chains of their fatty acids.
D) They have fewer fatty acids per fat molecule than do saturated fats.
E) They contain more hydrogen than do saturated fats having the same number of carbon atoms.
A) They are more common in animals.
B) They are generally liquid at room temperature.
C) They have no double bonds in the carbon chains of their fatty acids.
D) They have fewer fatty acids per fat molecule than do saturated fats.
E) They contain more hydrogen than do saturated fats having the same number of carbon atoms.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
You isolate a compound that is insoluble in water, has alternating single and double bonds, and has a bright orange color. You correctly conclude that this compound is a:
A) protein.
B) nucleic acid.
C) polysaccharide.
D) steroid.
E) carotenoid.
A) protein.
B) nucleic acid.
C) polysaccharide.
D) steroid.
E) carotenoid.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Glucose and fructose are ____ because they have identical molecular formulas but their atoms are arranged differently.
A) polar
B) tertiary
C) enantiomers
D) structural isomers
E) geometric isomers
A) polar
B) tertiary
C) enantiomers
D) structural isomers
E) geometric isomers

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
How many electron pairs are shared between carbon 2 and 3 in the accompanying figure? 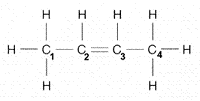
A) one
B) one and a half
C) two
D) three
E) four

A) one
B) one and a half
C) two
D) three
E) four

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
When a nucleic acid undergoes hydrolysis, the resulting subunits are:
A) fatty acids.
B) amino acids.
C) nucleotides.
D) carotenoids.
E) monosaccharides.
A) fatty acids.
B) amino acids.
C) nucleotides.
D) carotenoids.
E) monosaccharides.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Which description illustrates the tertiary structure of a protein molecule?
A) bonding of two amino acids to form a dipeptide
B) folding of a peptide chain to form an alpha helix
C) association of several polypeptide chains by weak bonds
D) order in which amino acids are joined in a peptide chain
E) three-dimensional shape of an individual polypeptide chain
A) bonding of two amino acids to form a dipeptide
B) folding of a peptide chain to form an alpha helix
C) association of several polypeptide chains by weak bonds
D) order in which amino acids are joined in a peptide chain
E) three-dimensional shape of an individual polypeptide chain

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Which statement best summarizes the differences between RNA and DNA?
A) RNA is a single-stranded form of DNA.
B) DNA is a polymer and RNA is a monomer.
C) RNA is a protein and DNA is a nucleic acid.
D) DNA is the primary energy currency of all cells.
E) DNA comprises the genes, while RNA is a direct participant in the process of protein synthesis.
A) RNA is a single-stranded form of DNA.
B) DNA is a polymer and RNA is a monomer.
C) RNA is a protein and DNA is a nucleic acid.
D) DNA is the primary energy currency of all cells.
E) DNA comprises the genes, while RNA is a direct participant in the process of protein synthesis.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
Figure 3-3 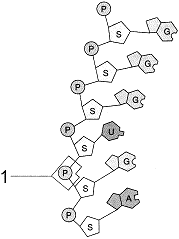 The molecular fragment (the G, U and A's) represented in the accompanying figure is:
The molecular fragment (the G, U and A's) represented in the accompanying figure is:
A) ATP.
B) RNA.
C) DNA.
D) a nucleotide.
E) a polysaccharide.
 The molecular fragment (the G, U and A's) represented in the accompanying figure is:
The molecular fragment (the G, U and A's) represented in the accompanying figure is:A) ATP.
B) RNA.
C) DNA.
D) a nucleotide.
E) a polysaccharide.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
At which level of protein structure are peptide bonds most important?
A) primary
B) secondary
C) tertiary
D) quaternary
E) globular
A) primary
B) secondary
C) tertiary
D) quaternary
E) globular

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
What is the purpose of molecular chaperones?
A) To transfer an amino acid
B) To attach a carboxyl group
C) To straighten other molecular proteins
D) To strengthen the tertiary structure of a protein
E) To assist the folding of other molecular proteins
A) To transfer an amino acid
B) To attach a carboxyl group
C) To straighten other molecular proteins
D) To strengthen the tertiary structure of a protein
E) To assist the folding of other molecular proteins

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Figure 3-3 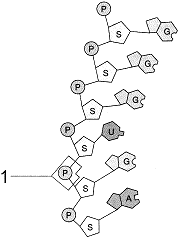 What type of connection exists between the atoms at the point labeled 1 in the accompanying figure?
What type of connection exists between the atoms at the point labeled 1 in the accompanying figure?
A) peptide bond
B) disulfide bond
C) hydrogen bond
D) glycoside linkage
E) phosphodiester linkage
 What type of connection exists between the atoms at the point labeled 1 in the accompanying figure?
What type of connection exists between the atoms at the point labeled 1 in the accompanying figure?A) peptide bond
B) disulfide bond
C) hydrogen bond
D) glycoside linkage
E) phosphodiester linkage

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
Figure 3-2 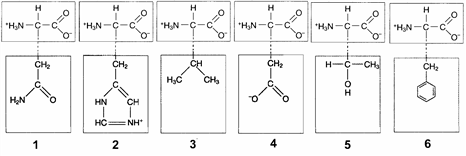 In the accompanying figure, hydrophobic interactions would occur between the R groups of which two amino acids?
In the accompanying figure, hydrophobic interactions would occur between the R groups of which two amino acids?
A) 1 and 4
B) 2 and 5
C) 3 and 6
D) 2 and 4
E) 3 and 5
 In the accompanying figure, hydrophobic interactions would occur between the R groups of which two amino acids?
In the accompanying figure, hydrophobic interactions would occur between the R groups of which two amino acids?A) 1 and 4
B) 2 and 5
C) 3 and 6
D) 2 and 4
E) 3 and 5

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Which functional group forms bridges to help stabilize a protein's quaternary structure?
A) amino
B) carbonyl
C) hydroxyl
D) phosphate
E) sulfhydryl
A) amino
B) carbonyl
C) hydroxyl
D) phosphate
E) sulfhydryl

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
Which pair matches the correct macromolecule with the bond that joins its subunits?
A) protein − ester linkage
B) steroid − peptide bond
C) polysaccharide − peptide bond
D) triacylglycerol − glycosidic linkage
E) nucleic acid − phosphodiester linkage
A) protein − ester linkage
B) steroid − peptide bond
C) polysaccharide − peptide bond
D) triacylglycerol − glycosidic linkage
E) nucleic acid − phosphodiester linkage

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
What is the purpose of regulatory proteins?
A) To store nutrients
B) To defend against foreign invaders
C) To catalyze a specific chemical reaction
D) To control the expression of specific genes
E) To strengthen and protect cells and tissues
A) To store nutrients
B) To defend against foreign invaders
C) To catalyze a specific chemical reaction
D) To control the expression of specific genes
E) To strengthen and protect cells and tissues

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is responsible for the alpha-helical structure of proteins?
A) hydrogen bonds
B) ionic interactions
C) polar covalent bonds
D) hydrophobic interactions
E) nonpolar covalent bonds
A) hydrogen bonds
B) ionic interactions
C) polar covalent bonds
D) hydrophobic interactions
E) nonpolar covalent bonds

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
The primary difference between the amino acids commonly found in proteins is in their:
A) R or variable groups.
B) number of potassium groups.
C) number of phosphate groups.
D) number of carbonyl groups.
E) number of asymmetric carbons.
A) R or variable groups.
B) number of potassium groups.
C) number of phosphate groups.
D) number of carbonyl groups.
E) number of asymmetric carbons.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
Figure 3-2 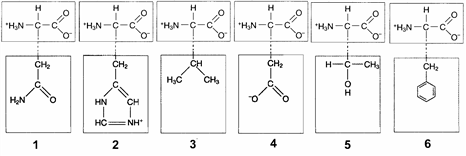 In the accompanying figure, ionic bonds would form between the R groups of which amino acids?
In the accompanying figure, ionic bonds would form between the R groups of which amino acids?
A) 1 and 3
B) 2 and 4
C) 3 and 5
D) 4 and 6
E) 3 and 6
 In the accompanying figure, ionic bonds would form between the R groups of which amino acids?
In the accompanying figure, ionic bonds would form between the R groups of which amino acids?A) 1 and 3
B) 2 and 4
C) 3 and 5
D) 4 and 6
E) 3 and 6

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Assume that the shaded portions of the molecule in the accompanying figure each represent different polypeptide chains. What does this represent? 
A) cellulose
B) a carotenoid
C) an amino acid
D) a steroid hormone
E) the quaternary structure of a protein

A) cellulose
B) a carotenoid
C) an amino acid
D) a steroid hormone
E) the quaternary structure of a protein

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
If tyrosine and isoleucine undergo condensation, where does the new bond form?
A) Between carbon of the R group and the nitrogen of the amino group
B) Between oxygen of the R group and the hydrogen of the amino group
C) Between carbon of the carboxyl group and the hydrogen of the R group
D) Between carbon of the carboxyl group and the hydrogen of the amino group
E) Between carbon of the carboxyl group and the nitrogen of the amino group
A) Between carbon of the R group and the nitrogen of the amino group
B) Between oxygen of the R group and the hydrogen of the amino group
C) Between carbon of the carboxyl group and the hydrogen of the R group
D) Between carbon of the carboxyl group and the hydrogen of the amino group
E) Between carbon of the carboxyl group and the nitrogen of the amino group

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
The helical coil shape of an α -helix fibrous protein provides what type of property to that protein?
A) rigidity
B) strength
C) elasticity
D) heat tolerance
E) water retention
A) rigidity
B) strength
C) elasticity
D) heat tolerance
E) water retention

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
The following amino acid would be characterized as ____ based on the chemical properties of its side chain. 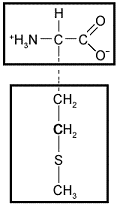
A) basic
B) acidic
C) nonpolar
D) hydrophilic
E) electrically charged

A) basic
B) acidic
C) nonpolar
D) hydrophilic
E) electrically charged

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Analysis of a certain polymer shows that it contains phosphate groups, ribose groups, and pyrimidines. Based on this information, what statement best describes this compound?
A) It is RNA.
B) It is DNA.
C) It is cylic AMP.
D) It is a polypeptide.
E) It is an inorganic compound.
A) It is RNA.
B) It is DNA.
C) It is cylic AMP.
D) It is a polypeptide.
E) It is an inorganic compound.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
What type of protein accelerates the thousands of different chemical reactions that take place in an organism?
A) enzyme
B) amino acid
C) transport protein
D) regulatory protein
E) protective protein
A) enzyme
B) amino acid
C) transport protein
D) regulatory protein
E) protective protein

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
Carotenoids are composed of isoprene subunits.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
What organic molecules is the primary structural component of cell membranes?
A) cellulose
B) glycogen
C) disaccharides
D) phospholipids
E) adenine triphosphate
A) cellulose
B) glycogen
C) disaccharides
D) phospholipids
E) adenine triphosphate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
A phosphate group is weakly acidic .
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Why is ATP important in living organisms?
A) It is easily converted to starch for long-term storage.
B) It can transfer some of its energy to other chemicals.
C) It is an important structural component of cell membranes.
D) Like all other nucleic acids, it stores hereditary information.
E) Like RNA, it acts as a source code for the formation of proteins.
A) It is easily converted to starch for long-term storage.
B) It can transfer some of its energy to other chemicals.
C) It is an important structural component of cell membranes.
D) Like all other nucleic acids, it stores hereditary information.
E) Like RNA, it acts as a source code for the formation of proteins.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
Identify the levels of organization for protein molecules, and list the type(s) of bond(s) involved in establishing each structural level.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
Fats high in unsaturated fatty acids tend to be solid at room temperature.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
A disaccharide is composed of two monosaccharides joined by a(n) glycosidic linkage .
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Identify three functions of proteins other than enzymes and briefly discuss or describe each.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Which organic compound is not only responsible for energy storage, but also can provide thermal insulation?
A) lipids
B) proteins
C) nucleic acids
D) carbohydrates
E) monosaccharides
A) lipids
B) proteins
C) nucleic acids
D) carbohydrates
E) monosaccharides

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is a purine base found in nucleotides?
A) uricil
B) steroid
C) guanine
D) cytosine
E) thymine
A) uricil
B) steroid
C) guanine
D) cytosine
E) thymine

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
What does the term "functional group" mean in reference to the structure of organic molecules? Identify two types of functional groups and describe their chemical properties.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
Which lipid can be identified by its isoprene units?
A) fats
B) steroids
C) carotenoids
D) amino acids
E) phospholipids
A) fats
B) steroids
C) carotenoids
D) amino acids
E) phospholipids

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Since a carbon atom has 4 valence electrons, it can complete its valence shell by forming a total of 4 hydrogen bonds.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Chitin is a polymer composed of N-acetyl glucosamine monomers.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Compare and contrast the structure, physical characteristics, and biological functions of two of the following: fats, steroids, and phospholipids.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
A(n) essential amino acid is one that the body cannot synthesize in sufficient amounts.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
When glucose and fructose undergo condensation, maltose is produced as a product.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
The carboxyl group can exist in an ionized form and also in a nonionized form.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
Condensation and hydrolysis reactions are catalyzed by the same enzymes..
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
By definition, geometric isomers are mirror images of each other.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
A beta-pleated sheet is an example of a protein's tertiary structure.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
Why is carbon ideally suited to be the "backbone" in molecules produced by living organisms?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
Methane, which is composed of carbon and hydrogen, lacks functional groups and is a gas at room temperature. Upon replacing one of the hydrogen atoms with a hydroxyl group, methane is converted to methanol, which is a liquid at room temperature. Explain the reason behind this difference in physical properties.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Match between columns
Premises:
contains purines and pyrimidines
contains purines and pyrimidines
contains purines and pyrimidines
contains purines and pyrimidines
cellulose
cellulose
cellulose
cellulose
most are nonpolar
most are nonpolar
most are nonpolar
most are nonpolar
consist of monomers having 20 different types
consist of monomers having 20 different types
consist of monomers having 20 different types
consist of monomers having 20 different types
Responses:
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid
lipid
carbohydrate
protein
nucleic acid

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
Cyclic AMP is a type of nucleotide .
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
A pyrimidine is a double -ring molecule.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
Molecular chaperones mediate the folding of other protein molecules.
__________________
__________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
Sickle cell anemia is a genetic disease caused by the replacement of one amino acid in the hemoglobin molecule. This replacement changes the shape and function of the hemoglobin protein in dramatic ways, which can sometimes be lethal. How can the substitution of one amino acid lead to such drastic results?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck



