Deck 12: Unsaturated Hydrocarbons
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 12: Unsaturated Hydrocarbons
1
What is the IUPAC name for the compound shown below? 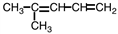
A)2-methyl-1,4-pentadiene
B)2-methyl-2,4-dipentene
C)4-methyl-1,3-pentadiene
D)4-methyl-2,4-pentadiene
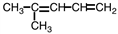
A)2-methyl-1,4-pentadiene
B)2-methyl-2,4-dipentene
C)4-methyl-1,3-pentadiene
D)4-methyl-2,4-pentadiene
4-methyl-1,3-pentadiene
2
Which of the following is the monomer used to produce Teflon ® ?
A)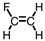
B)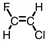
C)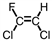
D)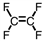
A)

B)

C)

D)


3
A portion of the structure of Acrilan ® is shown. What is the structure of the monomer? 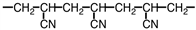
A)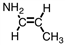
B)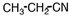
C)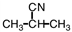
D)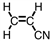

A)

B)
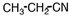
C)

D)


4
Which of the following compounds is trans -3-hexene?
A)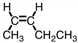
B)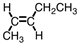
C)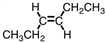
D)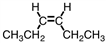
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
Select the major product that would result from the reaction below. CH3 − CH=CH2 + H2O 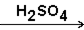
A)CH3CH(OH)CH3
B)CH3CH2CH2OH
C)CH3CH2CH3
D)CH3CH2CH2SO4
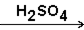
A)CH3CH(OH)CH3
B)CH3CH2CH2OH
C)CH3CH2CH3
D)CH3CH2CH2SO4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
Which is the formula for an alkene?
A)CH3CHCH2
B)CH3CH2CH2
C)CH3CH3CH2
D)More than one response is correct.
A)CH3CHCH2
B)CH3CH2CH2
C)CH3CH3CH2
D)More than one response is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following represents an addition reaction?
A)HX + C4H8 → C4H9X
B)X2 + C3H6 → C3H5X + HX
C)C4H8 → C4H6 + H2
D)more than one response is correct
A)HX + C4H8 → C4H9X
B)X2 + C3H6 → C3H5X + HX
C)C4H8 → C4H6 + H2
D)more than one response is correct

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
What number would be used to indicate the double bond position in the IUPAC name for CH3-CH2-CH=CH-CH3
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
Which characteristic relates to alkenes but not the other hydrocarbon families?
A)saturation
B)halogen substitution
C)double bonds
D)triple bonds
A)saturation
B)halogen substitution
C)double bonds
D)triple bonds

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the correct IUPAC name for the following compound? 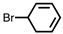
A)5-bromo-1,3-cyclohexadiene
B)6-bromo-1,3-cyclohexadiene
C)2-bromo-1,4-cyclohexadiene
D)3-bromo-1,5-cyclohexadiene

A)5-bromo-1,3-cyclohexadiene
B)6-bromo-1,3-cyclohexadiene
C)2-bromo-1,4-cyclohexadiene
D)3-bromo-1,5-cyclohexadiene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
Select the major product that would result from the reaction below. CH3CH2CH=CH2 + HBr → __________
A)CH3(CHBr)2CH3
B)CH2CH2CH2CH3
C)CH3CHBrCH2CH3
D)CH3CH2CH2CH2Br
A)CH3(CHBr)2CH3
B)CH2CH2CH2CH3
C)CH3CHBrCH2CH3
D)CH3CH2CH2CH2Br

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following can exhibit geometric isomerism?
A)1-propene
B)1,2,2-tribromoethene
C)2,3-dimethyl-2-butene
D)1-bromo-1-propene
A)1-propene
B)1,2,2-tribromoethene
C)2,3-dimethyl-2-butene
D)1-bromo-1-propene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
What reagent or reagents is required for the conversion of cyclohexene to cyclohexane?
A)HCl
B)H2O and H2SO4
C)H2 and H2SO4
D)H2 and Pt
A)HCl
B)H2O and H2SO4
C)H2 and H2SO4
D)H2 and Pt

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
What is the addition polymer produced from the monomer shown below? 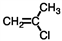
A)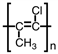
B)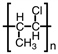
C)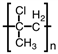
D)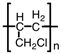

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is the polymer produced from CH3 − CH=CH − Cl?
A)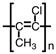
B)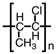
C)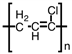
D)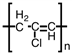
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
In the IUPAC name for the following compound, the -Br group is located at what position of the compound shown? CH3CHBrCH=CH2
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds could exhibit geometric isomerism?
A)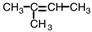
B)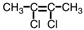
C)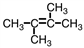
D)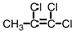
A)
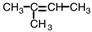
B)
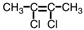
C)

D)
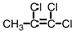

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the compounds below could correctly be called a cis compound?
A)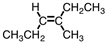
B)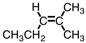
C)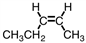
D)None of these
A)

B)

C)

D)None of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
Name a difference between a saturated and an unsaturated hydrocarbon.
A)Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated hydrocarbons include other atoms than just carbon and hydrogen.
B)Saturated hydrocarbons do not contain multiple bonds between carbons, but unsaturated hydrocarbons do contain multiple bonds.
C)Unsaturated hydrocarbons are flammable but saturated hydrocarbons are not.
D)Saturated hydrocarbons are essentially insoluble. Unsaturated hydrocarbons are soluble.
A)Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated hydrocarbons include other atoms than just carbon and hydrogen.
B)Saturated hydrocarbons do not contain multiple bonds between carbons, but unsaturated hydrocarbons do contain multiple bonds.
C)Unsaturated hydrocarbons are flammable but saturated hydrocarbons are not.
D)Saturated hydrocarbons are essentially insoluble. Unsaturated hydrocarbons are soluble.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name for the compound shown below? 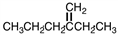
A)3-ethyl-1-pentene
B)2-ethyl-2-pentene
C)3-ethyl-3-pentene
D)2-ethyl-1-pentene

A)3-ethyl-1-pentene
B)2-ethyl-2-pentene
C)3-ethyl-3-pentene
D)2-ethyl-1-pentene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
Which is a difference between butene and cyclobutene?
A)They are isomers.
B)Cyclobutene has 2 double bonds, butene does not.
C)The location of the double bond is terminal in cyclobutene, but between interior carbons in butene.
D)Cyclobutene is missing more hydrogens than is butene.
A)They are isomers.
B)Cyclobutene has 2 double bonds, butene does not.
C)The location of the double bond is terminal in cyclobutene, but between interior carbons in butene.
D)Cyclobutene is missing more hydrogens than is butene.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
Acetylene is commercially useful as a fuel for torches and as
A)a starting material for plastics.
B)an industrial solvent.
C)an ingredient in pesticides.
D)a component in paint formulations.
A)a starting material for plastics.
B)an industrial solvent.
C)an ingredient in pesticides.
D)a component in paint formulations.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a useful organic solvent?
A)aniline
B)toluene
C)naphthalene
D)phenacetin
A)aniline
B)toluene
C)naphthalene
D)phenacetin

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
Identify the statement about lycopene that is true.
A)Lycopene is known as Vitamin C.
B)Lycopene gives watermelon their red color.
C)Raw tomatoes are a better source of lycopene than cooked tomatoes.
D)Lycopene should not be eaten with fatty foods.
A)Lycopene is known as Vitamin C.
B)Lycopene gives watermelon their red color.
C)Raw tomatoes are a better source of lycopene than cooked tomatoes.
D)Lycopene should not be eaten with fatty foods.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds is not considered aromatic?
A)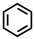
B)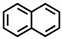
C)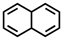
D)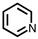
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
Select the product of the following reaction. 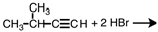
A)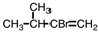
B)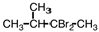
C)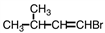
D)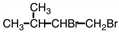
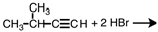
A)
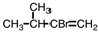
B)
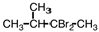
C)
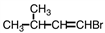
D)
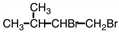

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is the correct name for the compound shown? 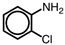
A)2-chlorophenol
B)2-chlorotoluene
C)2-chloroaniline
D)1-chloroaniline

A)2-chlorophenol
B)2-chlorotoluene
C)2-chloroaniline
D)1-chloroaniline

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is the correct IUPAC name for the compound CH2 − C ≡ C − CH2 − CH2 − Br?
A)4-bromopentyne
B)1-bromo-2-pentyne
C)1-bromo-3-pentyne
D)5-bromo-2-pentyne
A)4-bromopentyne
B)1-bromo-2-pentyne
C)1-bromo-3-pentyne
D)5-bromo-2-pentyne

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following structures violates the octet rule?
A)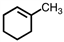
B)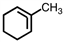
C)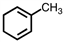
D)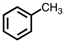
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
Which is a difference between butyne and cyclobutyne?
A)Cyclobutyne does not exist.
B)Butyne's multiple bond is interior, cyclobutyne is not between interior carbons.
C)Cyclobutyne burns much hotter than butyne because of the greater unsaturation.
D)None of these
A)Cyclobutyne does not exist.
B)Butyne's multiple bond is interior, cyclobutyne is not between interior carbons.
C)Cyclobutyne burns much hotter than butyne because of the greater unsaturation.
D)None of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
A major source of aromatic compounds is _____ .
A)coal tar
B)plants
C)animals
D)soils
A)coal tar
B)plants
C)animals
D)soils

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
Naphthalene is used as _____ .
A)an explosive
B)moth repellent
C)a pain reliever
D)a solvent
A)an explosive
B)moth repellent
C)a pain reliever
D)a solvent

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
What is the correct name for 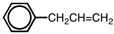 ?
?
A)3-phenyl-1-propene
B)1-phenyl-1-propene
C)1-phenyl-2-propene
D)3-phenyl-2-propene
 ?
?A)3-phenyl-1-propene
B)1-phenyl-1-propene
C)1-phenyl-2-propene
D)3-phenyl-2-propene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
The addition of two moles of hydrogen to an alkyne produces an _____ .
A)alkane
B)alkene
C)aromatic
D)alkyl halide
A)alkane
B)alkene
C)aromatic
D)alkyl halide

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
Which is the formula for an alkyne?
A)CH3CH2CCH2
B)CH3CH2CH2CH3
C)CH3CH2CCH
D)CH3CH2CCH2
A)CH3CH2CCH2
B)CH3CH2CH2CH3
C)CH3CH2CCH
D)CH3CH2CCH2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
What is the characteristic of aromatic compounds that is responsible for them being named aromatic compounds?
A)The compounds have a pleasant smell.
B)These compounds contain a benzene ring or structural relative.
C)A requirement is to contain a hydrocarbon chain that is either saturated or unsaturated and at least 3 carbons long.
D)There is more than one correct response.
A)The compounds have a pleasant smell.
B)These compounds contain a benzene ring or structural relative.
C)A requirement is to contain a hydrocarbon chain that is either saturated or unsaturated and at least 3 carbons long.
D)There is more than one correct response.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
The benzene ring as a branch is called a _____ group.
A)hexyl
B)benzyl
C)phenol
D)phenyl
A)hexyl
B)benzyl
C)phenol
D)phenyl

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
Which is a characteristic of alkenes and alkynes, but not a characteristic of alkanes?
A)Alkynes are not flammable, the others are flammable.
B)Alkenes all have a scent similar to the aromatic compounds, but the alkanes and alkenes have a scent that is extremely sharp.
C)Alkanes have only single bonds between carbons.
D)There is more than one correct response.
A)Alkynes are not flammable, the others are flammable.
B)Alkenes all have a scent similar to the aromatic compounds, but the alkanes and alkenes have a scent that is extremely sharp.
C)Alkanes have only single bonds between carbons.
D)There is more than one correct response.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
Another acceptable name for 1-ethyl-3-methylbenzene is _____ .
A)m -ethylmethyltoluene
B)o -ethylmethyltoluene
C)p -ethylmethyltoluene
D)m -ethyltoluene
A)m -ethylmethyltoluene
B)o -ethylmethyltoluene
C)p -ethylmethyltoluene
D)m -ethyltoluene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
Which is a difference between butane and butene?
A)butane burns and butene does not
B)the presence of a double bond
C)they are isomers
D)the presence of a triple bond
A)butane burns and butene does not
B)the presence of a double bond
C)they are isomers
D)the presence of a triple bond

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
Name the following compound. 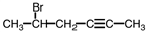
A)5-bromo-2-hexyne
B)bromo-4-hexyne
C)1-bromo-1-methyl-3-pentyne
D)none of these are correct
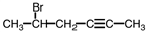
A)5-bromo-2-hexyne
B)bromo-4-hexyne
C)1-bromo-1-methyl-3-pentyne
D)none of these are correct

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
How many pi bonds are in the following two molecules, respectively from left to right? 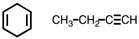
A)2, 1
B)1, 2
C)2, 2
D)4, 3
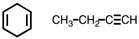
A)2, 1
B)1, 2
C)2, 2
D)4, 3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
Indicate the hybridization on each of the carbon atoms designated by a number in the following molecule. 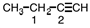
A)1 - sp , 2 - sp 2
B)1 - sp3 , 2 - sp 2
C)1 - sp2 , 2 - sp 3
D)1 - sp3 , 2 - sp
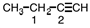
A)1 - sp , 2 - sp 2
B)1 - sp3 , 2 - sp 2
C)1 - sp2 , 2 - sp 3
D)1 - sp3 , 2 - sp

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
Indicate the geometry around each of the carbon atoms in the following molecule. 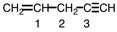
A)1 - triangular, 2- tetrahedral, 3 - linear
B)1 - linear, 2- tetrahedral, 3 - triangular
C)1 - tetrahedral, 2- tetrahedral, 3 - linear
D)1 - triangular, 2- linear, 3 - tetrahedral
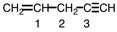
A)1 - triangular, 2- tetrahedral, 3 - linear
B)1 - linear, 2- tetrahedral, 3 - triangular
C)1 - tetrahedral, 2- tetrahedral, 3 - linear
D)1 - triangular, 2- linear, 3 - tetrahedral

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
What type of hybridization is associated with the carbons in ethene?
A)sp
B)sp 2
C)sp 3
D)sp 4
A)sp
B)sp 2
C)sp 3
D)sp 4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
Name the following aromatic compound. 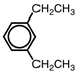
A)1,5-diethylbenzene
B)p -diethylbenzene
C)o -diethylbenzene
D)1,3-diethylbenzene

A)1,5-diethylbenzene
B)p -diethylbenzene
C)o -diethylbenzene
D)1,3-diethylbenzene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
According to Markovnikov's rule, when 3-methyl-1-butene undergoes and addition reaction with HCl, the chlorine will end up on which main chain carbon?
A)#1
B)#2
C)#3
D)#4
A)#1
B)#2
C)#3
D)#4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
The ability to detect light is based, in part, to a change of one from of retinal to another. Specifically, what is this change?
A)a cis to trans conversion
B)a trans to cis conversion
C)A hydrogenation reaction
D)A dehydrogenation reaction
A)a cis to trans conversion
B)a trans to cis conversion
C)A hydrogenation reaction
D)A dehydrogenation reaction

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following compounds is not possible?
A)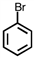
B)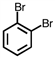
C)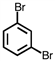
D)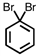
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following could exhibit cis\trans isomerism?
A)propene
B)1,2-dichloropropene
C)1-butene
D)2-butene
A)propene
B)1,2-dichloropropene
C)1-butene
D)2-butene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is a reasonable representation of an sp2 hybridized orbital?
A)
B)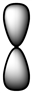
C)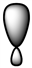
D)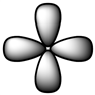
A)

B)
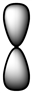
C)
D)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
What type of hybridization is associated with alkyne bonding?
A)sp
B)sp2
C)sp3
D)sp4
A)sp
B)sp2
C)sp3
D)sp4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
Poly (vinyl chloride), PVC, is used for water pipes and synthetic leather. What is the monomer of the PVC polymer shown below? 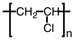
A)
B)
C)
D)

A)

B)

C)
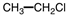
D)
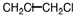

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
Starting with cyclopentene, indicate which of the following reactants would produce the product listed.
A)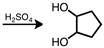
B)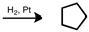
C)
D)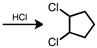
A)

B)
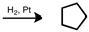
C)

D)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following will not reduce your cancer risk?
A)not smoking
B)being active
C)maintaining proper weight
D)cooking meats at high temperatures
A)not smoking
B)being active
C)maintaining proper weight
D)cooking meats at high temperatures

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
What would the reaction of hydrogen fluoride with ethene be an example of?
A)hydration
B)halogenation
C)hydrohalogenation
D)fluorination
A)hydration
B)halogenation
C)hydrohalogenation
D)fluorination

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
The following compound is a(n)____. 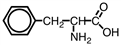
A)vitamin
B)industrial solvent
C)amino acid
D)monomer for polystyrene

A)vitamin
B)industrial solvent
C)amino acid
D)monomer for polystyrene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
Lycopene has been shown to prevent certain types of cancer. Which of the following is not a good source of lycopene?
A)tomatoes
B)pink grapefruit
C)guava
D)green beans
A)tomatoes
B)pink grapefruit
C)guava
D)green beans

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
Color is a property associated with which type of hydrocarbon?
A)alkanes
B)alkenes
C)alkynes
D)cycloalkanes
A)alkanes
B)alkenes
C)alkynes
D)cycloalkanes

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following accurately depicts o -iodotoluene?
A)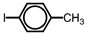
B)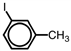
C)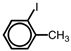
D)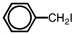
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
Benzene is an alkene with more than one multiple bond.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
The reaction of bromine with an alkene can be detected by which of the following?
A)formation of hydrogen gas
B)loss of bromine solution color
C)precipitate formation
D)color change from red to green
A)formation of hydrogen gas
B)loss of bromine solution color
C)precipitate formation
D)color change from red to green

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
An alkene with one multiple bond can be converted to an alkane by hydration.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
The general formula for an alkene is CnH2n.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
The general formula for an alkyne is CnH2n.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
The addition of bromine to an alkene results in an alkane because one bond of the multiple bond is broken.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
Two moles of hydrogen gas would be required to convert one mole of 2-butyne into butane.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
CH3CH2CH2CH2 is the formula for a saturated hydrocarbon.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
2-butyne can exist as cis - and trans - isomers.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
Alkenes must have at least two carbon atoms.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
Alkenes can only have one double bond.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
Polymers are compounds that are composed of repeating units chemically bound to each other.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
The physical properties of alkynes are very different from those of alkenes.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
A characteristic of alkynes is a region of strong polarity caused by the multiple bond.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
Phenyl is the name given to the ion produced when benzene loses one hydrogen, making it a substituent.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
One of the halogenation reactions occurs when a halogen, a member of group VIIA, reacts with alkene.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
Markovnikov's rule indicates that in the addition of H-X to an alkene, the hydrogen becomes attached to the carbon atom that is already bonded to more hydrogens.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
The same substances which add to double bonds can add to triple bonds.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following can be used to represent a phenyl branch when drawing an organic structure?
A)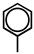
B)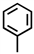
C)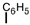
D)More than one answer is correct.
A)

B)

C)
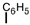
D)More than one answer is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
Cyclic compounds do not undergo halogenation reactions.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck



