Deck 5: Chemical Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 5: Chemical Bonding
1
Which of these is the most likely identity of element A in the Lewis dot structure?
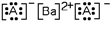
A)Ca
B)K
C)N
D)Cl
E)O
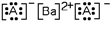
A)Ca
B)K
C)N
D)Cl
E)O
Cl
2
Which of these is the correct Lewis dot structure for sulfur?
A)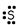
B)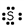
C)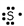
D)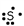
E)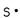
A)
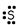
B)
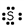
C)
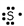
D)
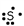
E)
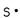
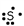
3
Which of these will not form a positive ion in an ionic compound?
A)Ba
B)Rb
C)P
D)Sn
E)Mg
A)Ba
B)Rb
C)P
D)Sn
E)Mg
P
4
What does the V in VSEPR theory stand for?
A)Very
B)Variable
C)Valence
D)Vanadium
E)Volume
A)Very
B)Variable
C)Valence
D)Vanadium
E)Volume

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these substances is\are covalent compound(s)?
I. CaO
II. H20
III. PF3
IV. CH4
A)I and II
B)II and III
C)II and IV
D)II, III, and IV
E)II only
I. CaO
II. H20
III. PF3
IV. CH4
A)I and II
B)II and III
C)II and IV
D)II, III, and IV
E)II only

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
An element which has two valence electrons as an atom will most likely:
A)lose 2 electrons to become negatively charged and combine with a cation.
B)lose 2 electrons to become positively charged and combine with an anion.
C)gain 2 electrons to become negatively charged and combine with a cation.
D)gain 2 electrons to become positively charged and combine with an anion.
E)All of the above can occur.
A)lose 2 electrons to become negatively charged and combine with a cation.
B)lose 2 electrons to become positively charged and combine with an anion.
C)gain 2 electrons to become negatively charged and combine with a cation.
D)gain 2 electrons to become positively charged and combine with an anion.
E)All of the above can occur.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these is the correct Lewis dot structure for carbon monoxide?
A)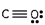
B)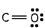
C)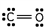
D)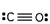
E)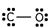
A)
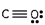
B)
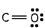
C)
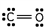
D)
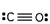
E)
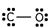

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these is the correct formula for aluminum oxide?
A)AlO
B)Al2O
C)AlO2
D)Al2O3
E)Al2O2
A)AlO
B)Al2O
C)AlO2
D)Al2O3
E)Al2O2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
Ionic bonds are formed when electrons are ____.
A)transferred
B)split
C)shared
D)destroyed
E)heated
A)transferred
B)split
C)shared
D)destroyed
E)heated

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
Which of these is the correct formula for sodium phosphide?
A)NaP
B)NaP2
C)Na3P
D)NaP3
E)Na3P2
A)NaP
B)NaP2
C)Na3P
D)NaP3
E)Na3P2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is true for sodium?
A)It is most stable as an atom.
B)It is most stable as a negatively charged ion.
C)It is most stable as a positively charged ion.
D)All forms of sodium are very stable.
E)All forms of sodium are very unstable.
A)It is most stable as an atom.
B)It is most stable as a negatively charged ion.
C)It is most stable as a positively charged ion.
D)All forms of sodium are very stable.
E)All forms of sodium are very unstable.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
Which of these substances is\are ionic compound(s)?
I. NCl3
II. MgF2
III. AlBr3
IV. CH4
A)I and II
B)II and III
C)II and IV
D)II, III, and IV
E)II only
I. NCl3
II. MgF2
III. AlBr3
IV. CH4
A)I and II
B)II and III
C)II and IV
D)II, III, and IV
E)II only

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
Which of these is not a fundamental postulate of bonding proposed by Lewis?
A)Valence electrons are shared in covalent bonding.
B)The valence shell is involved in chemical bonding.
C)Atoms bond to achieve a more stable configuration.
D)Atoms bond to achieve an octet of electrons in the valence shell.
E)A stable electron configuration has six electrons in the valence shell.
A)Valence electrons are shared in covalent bonding.
B)The valence shell is involved in chemical bonding.
C)Atoms bond to achieve a more stable configuration.
D)Atoms bond to achieve an octet of electrons in the valence shell.
E)A stable electron configuration has six electrons in the valence shell.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
Which of these is the correct Lewis dot structure for chlorine?
A)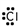
B)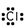
C)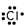
D)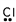
E)
A)
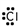
B)
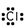
C)
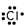
D)
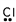
E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these is the most likely identity of element A in the Lewis dot structure?
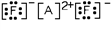
A)Ca
B)Li
C)Al
D)Cs
E)P
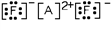
A)Ca
B)Li
C)Al
D)Cs
E)P

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
How many total valence electrons are there in the Lewis structure of NO3 − 1?
A)32
B)30
C)20
D)22
E)None of these.
A)32
B)30
C)20
D)22
E)None of these.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these is the correct formula for strontium iodide?
A)SrI
B)SrI2
C)Sr2I
D)SrI3
E)Sr2I2
A)SrI
B)SrI2
C)Sr2I
D)SrI3
E)Sr2I2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Covalent bonds are formed when electrons are ____.
A)transferred
B)split
C)shared
D)destroyed
E)heated
A)transferred
B)split
C)shared
D)destroyed
E)heated

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
The total number of valence electrons in NO2 −1 is
A)16
B)17
C)18
D)none of the above
A)16
B)17
C)18
D)none of the above

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
Which of these theories is utilized in the prediction of molecular shapes?
A)Dalton's Theory
B)VSEPR Theory
C)Bohr's Atomic Theory
D)Lewis Bonding Theory
E)Einstein's Theory of Relativity
A)Dalton's Theory
B)VSEPR Theory
C)Bohr's Atomic Theory
D)Lewis Bonding Theory
E)Einstein's Theory of Relativity

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
How many lone pairs of electrons are around the central atom in PCl3?
A)0
B)1
C)2
D)3
E)20
A)0
B)1
C)2
D)3
E)20

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
Which of these molecules contains a triple bond?
A)PCl3
B)N2
C)SO2
D)H2O
E)CO2
A)PCl3
B)N2
C)SO2
D)H2O
E)CO2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
Barium fluoride is often used in glass manufacturing. Which of these is the correct formula and bonding type for barium fluoride?
A)BaF, ionic
B)BaF, covalent
C)BaF2, ionic
D)BaF2, covalent
E)Ba2F, ionic
A)BaF, ionic
B)BaF, covalent
C)BaF2, ionic
D)BaF2, covalent
E)Ba2F, ionic

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these is the correct Lewis dot structure for sulfur trioxide?
A)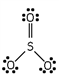
B)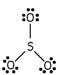
C)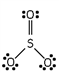
D)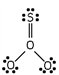
E)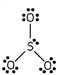
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is true about ozone?
A)Its Lewis structure is best represented with resonance structures.
B)It reduces the amount of ultraviolet radiation that reached the earth's surface.
C)It can be broken down by ultraviolet radiation.
D)All of the above.
E)None of the above.
A)Its Lewis structure is best represented with resonance structures.
B)It reduces the amount of ultraviolet radiation that reached the earth's surface.
C)It can be broken down by ultraviolet radiation.
D)All of the above.
E)None of the above.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
Which of these is the correct Lewis dot structure for nitrogen, N2?
A)
B)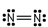
C)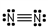
D)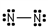
A)

B)
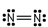
C)
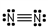
D)
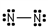

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
Which of these molecules contains two double bonds?
A)PCl3
B)N2
C)SO2
D)H2O
E)CO2
A)PCl3
B)N2
C)SO2
D)H2O
E)CO2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
How many lone pairs of electrons are around the central atom in CH4?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
How many lone pairs of electrons are around the central atom in CO2?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
How many bonding pairs of electrons are around the central atom in CO2?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
Which of these molecules contains 1 double bond and 1 single bond?
A)PCl3
B)NH3
C)SO2
D)H2O
E)CO2
A)PCl3
B)NH3
C)SO2
D)H2O
E)CO2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
Identify the fourth-row element X that forms the following ion:
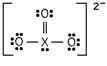
A)Ge
B)As
C)Se
D)Kr
E)Br
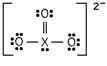
A)Ge
B)As
C)Se
D)Kr
E)Br

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
Which of these molecules contains a triple bond?
A)NH3
B)OCl2
C)C2H2
D)H2O
E)MgO
A)NH3
B)OCl2
C)C2H2
D)H2O
E)MgO

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
Identify the third-row element X which forms the following compound:
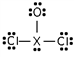
A)S
B)Cl
C)P
D)Si
E)Al

A)S
B)Cl
C)P
D)Si
E)Al

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
What is the molecular geometry of NH3?
A)Tetrahedral
B)Bent
C)Trigonal pyramidal
D)Trigonal planar
E)Linear
A)Tetrahedral
B)Bent
C)Trigonal pyramidal
D)Trigonal planar
E)Linear

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
How many lone pairs of electrons are around the central atom in H2O?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
How many bonding pairs of electrons are around the central atom in HCN?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
The molecular geometry of a two atom molecule:
A)is always bent.
B)is always tetrahedral.
C)is always linear.
D)depends on the atoms involved.
A)is always bent.
B)is always tetrahedral.
C)is always linear.
D)depends on the atoms involved.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
Which of these molecules will have a tetrahedral molecular geometry?
A)NH3
B)N2O
C)C2H2
D)CH3Cl
E)MgO
A)NH3
B)N2O
C)C2H2
D)CH3Cl
E)MgO

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
How many lone pairs of electrons are around the central atom in SO2?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
Which of these molecules will have a linear electron geometry and a trigonal pyramidal molecular geometry?
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)None of these.
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)None of these.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
Which of these molecules will have a trigonal planar electron geometry and a bent molecular geometry?
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)SO2
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)SO2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
Which of these molecules will have a tetrahedral electron geometry and a bent molecular geometry?
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)SO2
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)SO2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
What is the molecular geometry of NH4+?
A)Trigonal planar
B)Tetrahedral
C)Trigonal pyramidal
D)Bent
A)Trigonal planar
B)Tetrahedral
C)Trigonal pyramidal
D)Bent

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
Which of these would be expected to have a linear molecular geometry?
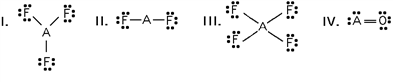
A)I and II
B)II and IV
C)I, II, and IV
D)I, and III
E)IV only

A)I and II
B)II and IV
C)I, II, and IV
D)I, and III
E)IV only

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
Which of these is the correct molecular geometry of BF3?
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
Which of these is the correct molecular geometry of CO2?
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
Which of these is the correct molecular geometry of PCl3?
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
Which of these is the correct molecular geometry of SO2?
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
Which of these molecules will have a linear electron geometry and a linear molecular geometry?
A)PCl3
B)H2O
C)CO
D)CH3Cl
E)BF3
A)PCl3
B)H2O
C)CO
D)CH3Cl
E)BF3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
Which of these molecules will have a trigonal pyramidal molecular geometry?
A)PCl3
B)N2O
C)C2H2
D)CH3Cl
E)SO2
A)PCl3
B)N2O
C)C2H2
D)CH3Cl
E)SO2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
Which of these would be expected to have a trigonal planar electron geometry?
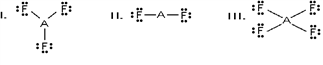
A)I and II
B)II and III
C)I, II, and III
D)I and III
E)I only
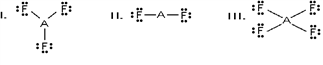
A)I and II
B)II and III
C)I, II, and III
D)I and III
E)I only

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
Electronegativity is:
A)the ability of an atom to form positively charged ions.
B)the ability of an atom to form negatively charged ions.
C)also called a dipole
D)the ability of an atom to attract bonding electrons.
E)the ability of an atom to form polar molecules.
A)the ability of an atom to form positively charged ions.
B)the ability of an atom to form negatively charged ions.
C)also called a dipole
D)the ability of an atom to attract bonding electrons.
E)the ability of an atom to form polar molecules.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
Which of these theories is utilized in the prediction of molecular polarity?
A)Dalton's Theory
B)VSEPR Theory
C)Electronegativity
D)Einstein's Relativity Theory
E)Both B and C
A)Dalton's Theory
B)VSEPR Theory
C)Electronegativity
D)Einstein's Relativity Theory
E)Both B and C

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
Which of these is the correct molecular geometry of CCl4?
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent
A)Linear
B)Trigonal planar
C)Tetrahedral
D)Trigonal pyramidal
E)Bent

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following results in bent molecular geometry?
A)4 bonding groups and no lone pair on the central atom
B)2 bonding groups and 1 lone pair on the central atom
C)2 bonding groups and 2 lone pairs on the central atom
D)Both B and C
E)Both A and C
A)4 bonding groups and no lone pair on the central atom
B)2 bonding groups and 1 lone pair on the central atom
C)2 bonding groups and 2 lone pairs on the central atom
D)Both B and C
E)Both A and C

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
A molecule with tetrahedral electron geometry could have which of the following molecular geometry?
A)Tetrahedral
B)Trigonal pyramidal
C)Bent
D)All of the above
A)Tetrahedral
B)Trigonal pyramidal
C)Bent
D)All of the above

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
Which of these molecules will have a tetrahedral electron geometry and a tetrahedral molecular geometry?
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)SO2
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)SO2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
The element with the highest electronegativity is _________.
A)fluorine
B)hydrogen
C)helium
D)neon
A)fluorine
B)hydrogen
C)helium
D)neon

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Which of these molecules will have a tetrahedral electron geometry and a trigonal pyramidal molecular geometry?
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)SO2
A)PCl3
B)H2O
C)C2H2
D)CH3Cl
E)SO2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
The [NO2] − molecule has ____ double bond(s), ____ single bond(s), ____ lone (nonbonding)pair(s)of electrons, and ____ resonance forms.
A)2, 0, 8, 2
B)1, 1, 8, 3
C)1, 1, 6, 2
D)0, 2, 10, 0
E)2, 0, 12, 2
A)2, 0, 8, 2
B)1, 1, 8, 3
C)1, 1, 6, 2
D)0, 2, 10, 0
E)2, 0, 12, 2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
Of the bonds below, the most polar bond would be:
A)C − C
B)C − N
C)C − O
D)C − F
A)C − C
B)C − N
C)C − O
D)C − F

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
Water:
A)is a polar molecule.
B)has tetrahedral electron geometry.
C)has bent molecular geometry.
D)has two lone pairs of electrons on the central atom.
E)All of the above.
A)is a polar molecule.
B)has tetrahedral electron geometry.
C)has bent molecular geometry.
D)has two lone pairs of electrons on the central atom.
E)All of the above.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
A nonpolar bond would most likely result when:
A)a metal bonds to a nonmetal.
B)a nonmetal bonds to a metal.
C)two identical nonmetal atoms bond.
D)an atom bonds to hydrogen.
A)a metal bonds to a nonmetal.
B)a nonmetal bonds to a metal.
C)two identical nonmetal atoms bond.
D)an atom bonds to hydrogen.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
Which of these is\are polar molecule(s)?
I. PCl3
II. H2O
III. CO2
IV. BF3
A)I only
B)I and II
C)I, II, and IV
D)II only
E)II and IV
I. PCl3
II. H2O
III. CO2
IV. BF3
A)I only
B)I and II
C)I, II, and IV
D)II only
E)II and IV

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
Which of these is a nonpolar molecule?
A)CO
B)H2O
C)O3
D)PCl3
E)SO3
A)CO
B)H2O
C)O3
D)PCl3
E)SO3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Element A has an electronegativity of 0.8 and element B has an electronegativity of 2.0. Which statement best describes the bonding in A3B?
A)The AB bond is largely covalent with a δ− on A.
B)The AB bond is largely covalent with a δ + on A.
C)The compound is largely ionic with no polar bonds.
D)The compound is largely covalent with no polar bonds.
E)There is insufficient information.
A)The AB bond is largely covalent with a δ− on A.
B)The AB bond is largely covalent with a δ + on A.
C)The compound is largely ionic with no polar bonds.
D)The compound is largely covalent with no polar bonds.
E)There is insufficient information.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
Which of these elements has the lowest electronegativity?
A)Ca
B)N
C)Se
D)P
E)O
A)Ca
B)N
C)Se
D)P
E)O

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
List the elements Na, K, Al, Cl, Cs in order of increasing electronegativity (from least to greatest).
A)Na < Cs < Al < K < Cl
B)Cs < Na < K < Cl < Al
C)Cl < Na < Al < K < Cs
D)Cl < Al < Na < K < Cs
E)Cs < K < Na < Al < Cl
A)Na < Cs < Al < K < Cl
B)Cs < Na < K < Cl < Al
C)Cl < Na < Al < K < Cs
D)Cl < Al < Na < K < Cs
E)Cs < K < Na < Al < Cl

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following molecules is nonpolar?
A)CO2
B)N2
C)CH4
D)CCl4
E)All of the above.
A)CO2
B)N2
C)CH4
D)CCl4
E)All of the above.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
Which of these elements has the highest electronegativity?
A)Ca
B)N
C)Se
D)P
E)O
A)Ca
B)N
C)Se
D)P
E)O

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
Which of these is\are polar molecule(s)with a tetrahedral electron geometry?
I. NCl3
II. H2O
III. CH3Cl
IV. SiH4
A)I only
B)I and III
C)I, II, and III
D)III only
E)II and III
I. NCl3
II. H2O
III. CH3Cl
IV. SiH4
A)I only
B)I and III
C)I, II, and III
D)III only
E)II and III

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
Which of these contain(s)polar bonds but is\are nonpolar molecule(s)?
I. NCl3
II. H2
III. CO2
IV. BF3
A)I only
B)I and III
C)III and IV
D)III only
E)I, III, and IV
I. NCl3
II. H2
III. CO2
IV. BF3
A)I only
B)I and III
C)III and IV
D)III only
E)I, III, and IV

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
List the elements Na, K, Al, Cl, Cs in order of decreasing electronegativity (from greatest to least).
A)Na > Cl > Al > K > Cs
B)Cs > Na > K > Cl > Al
C)Cl > Na > Al > K > Cs
D)Cl > Al > Na > K > Cs
E)Na > Cs > K > Al > Cl
A)Na > Cl > Al > K > Cs
B)Cs > Na > K > Cl > Al
C)Cl > Na > Al > K > Cs
D)Cl > Al > Na > K > Cs
E)Na > Cs > K > Al > Cl

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck



