Deck 9: Chemical Bonds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/119
Play
Full screen (f)
Deck 9: Chemical Bonds
1
Predict the formula of an ionic compound formed from barium and sulfur.
A) BaS
B) Ba2S
C) Ba S2
D) Ba3S
E) none of these
A) BaS
B) Ba2S
C) Ba S2
D) Ba3S
E) none of these
BaS
2
Consider the reaction of sodium metal with chlorine gas to form NaCl as shown below. How does each element in the compound NaCl attain an octet of electrons?
2 Na (s) + Cl2 (g)→2 NaCl (s)
A) A sodium atom and a chlorine atom share two electrons.
B) A sodium atom loses an electron and the chlorine atom accepts the electron.
C) A chlorine atom loses an electron and the sodium atom accepts the electron.
D) A sodium atom loses an electron and the chlorine atom loses seven electrons.
E) A sodium atom gains seven electrons and the chlorine atom loses seven electrons.
2 Na (s) + Cl2 (g)→2 NaCl (s)
A) A sodium atom and a chlorine atom share two electrons.
B) A sodium atom loses an electron and the chlorine atom accepts the electron.
C) A chlorine atom loses an electron and the sodium atom accepts the electron.
D) A sodium atom loses an electron and the chlorine atom loses seven electrons.
E) A sodium atom gains seven electrons and the chlorine atom loses seven electrons.
A sodium atom loses an electron and the chlorine atom accepts the electron.
3
Given the three statements below, which choice is correct?
I. The principal force holding a crystal of NaCl together is electrostatic (an attraction of positive and negative charges) in nature.
II. The Lewis symbol of sulfur is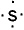
III. The Lewis symbol of carbon is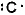
A) only I is true
B) II and III are true, I is false
C) all three are true
D) all three are false
E) none of these answers are correct
I. The principal force holding a crystal of NaCl together is electrostatic (an attraction of positive and negative charges) in nature.
II. The Lewis symbol of sulfur is
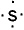
III. The Lewis symbol of carbon is
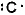
A) only I is true
B) II and III are true, I is false
C) all three are true
D) all three are false
E) none of these answers are correct
only I is true
4
Given the three statements below, pick the best answer.
I. The main forces that make ionic solids stable are the crystal lattice forces.
II. The main force holding covalent molecules together is the sharing of electrons between atoms.
III. The total number of valence electrons for CCl4 is 32.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only III is true
I. The main forces that make ionic solids stable are the crystal lattice forces.
II. The main force holding covalent molecules together is the sharing of electrons between atoms.
III. The total number of valence electrons for CCl4 is 32.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only III is true

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
5
Which atom shown below normally forms three covalent bonds when forming molecular compounds?
A) Cl
B) S
C) P
D) Si
E) Ar
A) Cl
B) S
C) P
D) Si
E) Ar

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
6
Which atom from the list below normally forms compounds with three covalent bonds to itself?
A) S
B) N
C) Mg
D) C
E) F
A) S
B) N
C) Mg
D) C
E) F

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
7
Which atom from the list below normally forms two covalent bonds when forming molecular compounds?
A) Ne
B) Si
C) N
D) S
E) Br
A) Ne
B) Si
C) N
D) S
E) Br

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
8
What is the correct Lewis dot symbol for a nitride ion , N3 - ?
A)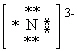
B)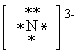
C)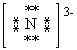
D)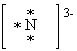
E)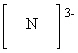
A)
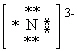
B)
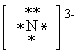
C)
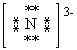
D)
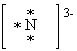
E)
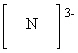

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
9
Which species listed below has the largest lattice energy?
A) NaCl
B) LiCl
C) LiF
D) KBr
E) KF
A) NaCl
B) LiCl
C) LiF
D) KBr
E) KF

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
10
A chemical property of hydrogen, H₂ , is that it reacts with oxygen, O₂, to produce water, H₂ O (a molecular compound), as shown below. How does each element in the compound H₂ O attain a Noble gas electronic configuration?
2 H₂ (g) + O₂(g) 2 H₂ O ( )
)
A) An oxygen atom loses two electrons and each hydrogen atom accepts an electron forming O2+ and two H - ions.
B) Each hydrogen atom loses an electron and the oxygen atom loses six electrons.
C) Each hydrogen atom loses an electron and the oxygen atom accepts the two electrons forming 2 H+ ions and one O2 - ion.
D) Each hydrogen atom gains seven electrons and the oxygen atom loses six electrons.
E) Each hydrogen atom and the oxygen atom share two electrons.
2 H₂ (g) + O₂(g) 2 H₂ O (
 )
)A) An oxygen atom loses two electrons and each hydrogen atom accepts an electron forming O2+ and two H - ions.
B) Each hydrogen atom loses an electron and the oxygen atom loses six electrons.
C) Each hydrogen atom loses an electron and the oxygen atom accepts the two electrons forming 2 H+ ions and one O2 - ion.
D) Each hydrogen atom gains seven electrons and the oxygen atom loses six electrons.
E) Each hydrogen atom and the oxygen atom share two electrons.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
11
What is the Electron Dot formula for the oxide ion, O2 - ?
A)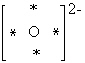
B)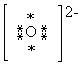
C)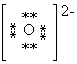
D)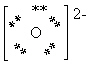
E)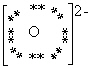
A)

B)
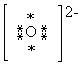
C)
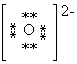
D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
12
What is(are) the driving force(s) for formation of an ionic compound from its elements?
A) A large amount of heat is released when two oppositely charged ions come together to form a crystal lattice.
B) Both elements in the compound attain a stable Noble gas electronic configuration through a complete transfer of electrons from the metal to the nonmetal.
C) Both elements in the compound attain a stable Noble gas electronic configuration through sharing of electrons.
D) Both a and b
E) Both a and c
A) A large amount of heat is released when two oppositely charged ions come together to form a crystal lattice.
B) Both elements in the compound attain a stable Noble gas electronic configuration through a complete transfer of electrons from the metal to the nonmetal.
C) Both elements in the compound attain a stable Noble gas electronic configuration through sharing of electrons.
D) Both a and b
E) Both a and c

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
13
Which species listed below has the largest lattice energy?
A) CaCl2
B) LiCl
C) BeO
D) CaO
E) CaS
A) CaCl2
B) LiCl
C) BeO
D) CaO
E) CaS

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
14
Which atom from the list below normally forms two covalent bonds ?
A) N
B) F
C) C
D) Br
E) O
A) N
B) F
C) C
D) Br
E) O

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
15
Which compound listed below should have the highest lattice energy?
A) LiCl
B) LiBr
C) NaBr
D) LiI
E) NaCl
A) LiCl
B) LiBr
C) NaBr
D) LiI
E) NaCl

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
16
Taking into account the size of ions and their respective charges, arrange the following three ionic compounds MgCl2, NaCl and MgO in order of increasing lattice energy .
A) MgCl2 < NaCl < MgO
B) MgCl2 < MgO < NaCl
C) NaCl < MgCl2 < MgO
D) NaCl < MgO < MgCl2
E) MgO < MgCl2 < NaCl
A) MgCl2 < NaCl < MgO
B) MgCl2 < MgO < NaCl
C) NaCl < MgCl2 < MgO
D) NaCl < MgO < MgCl2
E) MgO < MgCl2 < NaCl

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
17
A chemical property of magnesium metal, Mg (s), is that it reacts with oxygen, O2 (g), to produce magnesium oxide, MgO, as shown below. How does each element in the compound MgO attain an octet of electrons?
2 Mg (s) + O2 (g)→2 MgO (s)
A) A magnesium atom loses two electrons and the oxygen atom accepts the two electrons.
B) An oxygen atom loses two electrons and the magnesium atom accepts the two electrons.
C) A magnesium atom loses two electrons and the oxygen atom loses six electrons.
D) A magnesium atom gains six electrons and the oxygen atom loses six electrons.
E) A magnesium atom and an oxygen atom share two electrons.
2 Mg (s) + O2 (g)→2 MgO (s)
A) A magnesium atom loses two electrons and the oxygen atom accepts the two electrons.
B) An oxygen atom loses two electrons and the magnesium atom accepts the two electrons.
C) A magnesium atom loses two electrons and the oxygen atom loses six electrons.
D) A magnesium atom gains six electrons and the oxygen atom loses six electrons.
E) A magnesium atom and an oxygen atom share two electrons.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a false statement regarding the differences between ionic and covalent bonding?
A) Ionic bonds form from sharing electrons and covalent bonds form by a complete transfer of electrons from one atom to another.
B) The driving force for the formation of both ionic and molecular compounds is to attain a stable "Noble Gas" electronic configuration.
C) Ionic bonds form between a metal and a nonmetal whereas covalent bonds form between two nonmetals.
D) Ionic compounds exist as extended arrays of alternating cations and anions whereas covalently bonded compounds exist as discrete entities with weak forces of interaction among the molecules.
E) They are all true statements.
A) Ionic bonds form from sharing electrons and covalent bonds form by a complete transfer of electrons from one atom to another.
B) The driving force for the formation of both ionic and molecular compounds is to attain a stable "Noble Gas" electronic configuration.
C) Ionic bonds form between a metal and a nonmetal whereas covalent bonds form between two nonmetals.
D) Ionic compounds exist as extended arrays of alternating cations and anions whereas covalently bonded compounds exist as discrete entities with weak forces of interaction among the molecules.
E) They are all true statements.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
19
Given the three statements below, pick the best answer.
I. The Lewis symbol of boron is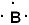
II. KCl should have a greater lattice energy than BeO.
III. The Lewis symbol Mg is correct for a magnesium atom.
A) all three are true
B) I and II are true, III is false
C) I and III are true, II is false
D) II and III are true, I is false
E) only III is true
I. The Lewis symbol of boron is
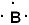
II. KCl should have a greater lattice energy than BeO.
III. The Lewis symbol Mg is correct for a magnesium atom.
A) all three are true
B) I and II are true, III is false
C) I and III are true, II is false
D) II and III are true, I is false
E) only III is true

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
20
Which species listed below has the largest lattice energy?
A) CaCl2
B) LiCl
C) MgF2
D) NaCl
E) SrBr2
A) CaCl2
B) LiCl
C) MgF2
D) NaCl
E) SrBr2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
21
The Lewis structure for formaldehyde (CH2O) is:
A)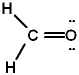
B)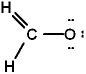
C)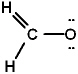
D)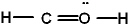
E) none of these
A)

B)

C)

D)
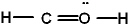
E) none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
22
The molecule H2NCH3 has:
A) 6 bonding pairs and 1 lone pair.
B) 7 bonding pairs and no lone pairs.
C) 5 bonding pairs and 2 lone pairs.
D) 5 bonding pairs and 1 lone pair.
E) some number of lone and bond pairs not described in a-d.
A) 6 bonding pairs and 1 lone pair.
B) 7 bonding pairs and no lone pairs.
C) 5 bonding pairs and 2 lone pairs.
D) 5 bonding pairs and 1 lone pair.
E) some number of lone and bond pairs not described in a-d.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
23
How many electrons (both lone and bond pairs) are shown in the correct Lewis structure of HClO?
A) 14
B) 12
C) 16
D) 20
E) none of these
A) 14
B) 12
C) 16
D) 20
E) none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
24
Which Lewis structure below obeys the octet rule for every atom in the structure of SO2 and uses the proper number of valence electrons?
A)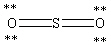
B)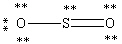
C)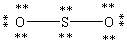
D)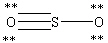
E)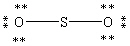
A)
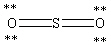
B)
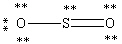
C)

D)
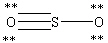
E)
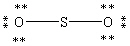

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
25
The molecule PH3 has:
A) 3 bonding pairs and 1 lone pair.
B) 3 bonding pairs and no lone pairs.
C) 3 bonding pairs and 2 lone pairs.
D) 4 bonding pairs and 1 lone pair.
E) some number of lone and bond pairs not described in a-d.
A) 3 bonding pairs and 1 lone pair.
B) 3 bonding pairs and no lone pairs.
C) 3 bonding pairs and 2 lone pairs.
D) 4 bonding pairs and 1 lone pair.
E) some number of lone and bond pairs not described in a-d.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
26
How many electrons (both lone and bond pairs) are shown in the Lewis diagram of CO32 -?
A) 20
B) 22
C) 24
D) 28
E) 30
A) 20
B) 22
C) 24
D) 28
E) 30

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
27
The molecule H2S has:
A) 2 bonding pairs and 3 lone pairs.
B) 2 bonding pairs and 2 lone pairs.
C) 3 bonding pairs and 1 lone pair.
D) 3 bonding pairs and 3 lone pairs.
E) none of these.
A) 2 bonding pairs and 3 lone pairs.
B) 2 bonding pairs and 2 lone pairs.
C) 3 bonding pairs and 1 lone pair.
D) 3 bonding pairs and 3 lone pairs.
E) none of these.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
28
Which Lewis structure below for CO2 obeys the octet rule for every atom in the structure and uses the proper number of valence electrons?
A)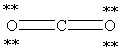
B)
C)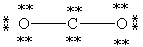
D)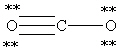
E)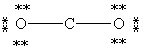
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
29
Given the three Lewis structures listed below, pick the best answer.
I.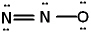
II.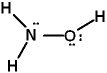
III.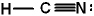
A) I and II are correct, III is incorrect
B) II and III are correct, I is incorrect
C) I and III are correct, II is incorrect
D) all three are correct
E) only II is correct
I.
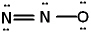
II.

III.
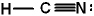
A) I and II are correct, III is incorrect
B) II and III are correct, I is incorrect
C) I and III are correct, II is incorrect
D) all three are correct
E) only II is correct

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
30
How many electrons (both lone and bond pairs) are used in the Lewis structure of C2O42 - ?
A) 30
B) 34
C) 32
D) 46
E) none of these
A) 30
B) 34
C) 32
D) 46
E) none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
31
How many electrons (both lone and bond pairs) are used in the Lewis diagram of CH2NH?
A) 16
B) 12
C) 14
D) 10
E) none of these
A) 16
B) 12
C) 14
D) 10
E) none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
32
Given the three Lewis structures below, pick the best answer.
I.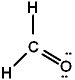
II.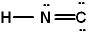
III.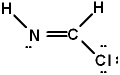
A) All three represent correct Lewis structures.
B) I and II represent correct Lewis structures, III does not
C) I and III represent correct Lewis structures, II does not
D) II and III represent correct Lewis structures, I does not
E) Only I represents a correct Lewis structure.
I.

II.
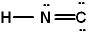
III.

A) All three represent correct Lewis structures.
B) I and II represent correct Lewis structures, III does not
C) I and III represent correct Lewis structures, II does not
D) II and III represent correct Lewis structures, I does not
E) Only I represents a correct Lewis structure.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
33
Which is the best Lewis structure of CH2FCO2H (connectivity correct as given)?
A)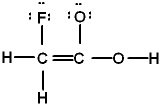
B)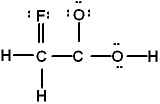
C)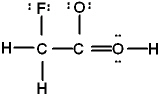
D)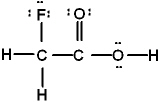
E) none of these is acceptable
A)

B)

C)

D)

E) none of these is acceptable

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
34
Lewis structures for three molecules are shown below (assume the connectivity is correct). Pick the best answer.
I.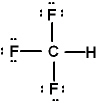
II.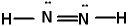
III.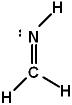
A) I and II are correct, III is not
B) I and III are correct, II is not
C) II and III are correct, I is not
D) all three are correct
E) only I is correct
I.

II.
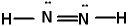
III.

A) I and II are correct, III is not
B) I and III are correct, II is not
C) II and III are correct, I is not
D) all three are correct
E) only I is correct

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
35
The molecule N2H4 (H2NNH2 connectivity) has in its correct Lewis structure a total of:
A) 4 bonds and 2 lone pairs
B) 5 bonds and 1 lone pair.
C) 4 bonds and 1 lone pair.
D) 5 bonds and 2 lone pairs.
E) none of these.
A) 4 bonds and 2 lone pairs
B) 5 bonds and 1 lone pair.
C) 4 bonds and 1 lone pair.
D) 5 bonds and 2 lone pairs.
E) none of these.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
36
How many electrons (both bond and lone pairs) are used in the Lewis structure of SO42 - ?
A) 18
B) 20
C) 22
D) 24
E) 32
A) 18
B) 20
C) 22
D) 24
E) 32

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
37
How many electrons (both bond and lone pairs) are used in the Lewis diagram of [NO3] - ?
A) 24
B) 20
C) 23
D) 25
E) none of these
A) 24
B) 20
C) 23
D) 25
E) none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
38
How many electrons (both lone and bond pairs) are used in the Lewis structure of NH3?
A) 10
B) 6
C) 8
D) 4
E) none of these
A) 10
B) 6
C) 8
D) 4
E) none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
39
The molecule H2O2 (H - O - O - H) has:
A) 4 bonding pairs and 3 lone pair.
B) 3 bonding pairs and 2 lone pairs.
C) 3 bonding pairs and 3 lone pairs.
D) 3 bonding pairs and 4 one pair.
E) some number of lone and bond pairs not described in a-d.
A) 4 bonding pairs and 3 lone pair.
B) 3 bonding pairs and 2 lone pairs.
C) 3 bonding pairs and 3 lone pairs.
D) 3 bonding pairs and 4 one pair.
E) some number of lone and bond pairs not described in a-d.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
40
The correct Lewis structure for Cl2CO is (the 2 Cl's and the O are bound to the C and not to each other):
A)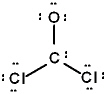
B)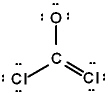
C)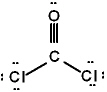
D)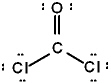
E) none of these
A)

B)

C)

D)

E) none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
41
Which Lewis structure below obeys the octet rule for every atom in the structure of SF2 and uses the proper number of valence electrons?
A)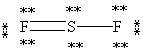
B)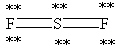
C)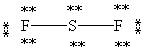
D)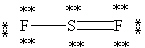
E)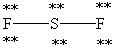
A)

B)
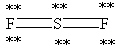
C)
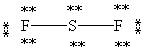
D)
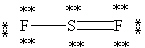
E)
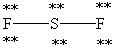

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
42
Which Lewis structure below for (NO3)1 - obeys the octet rule for every atom in the structure and uses the proper number of valence electrons?
A)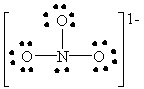
B)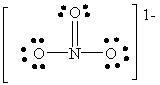
C)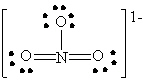
D)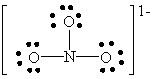
E)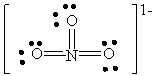
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
43
Which one of the following is the correct Lewis structure for HCN?
A)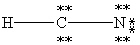
B)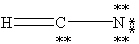
C)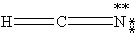
D)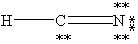
E)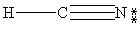
A)

B)
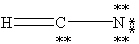
C)
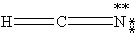
D)

E)
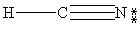

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
44
Which Lewis structure below obeys the octet rule for every atom in the structure of SO3 and uses the proper number of valence electrons?
A)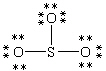
B)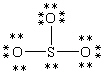
C)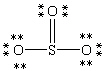
D)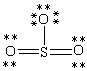
E)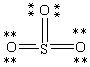
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
45
Which Lewis structure below obeys the octet rule for every atom in the structure of CO and uses the proper number of valence electrons?
A)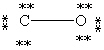
B)
C)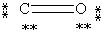
D)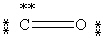
E)
A)

B)

C)

D)
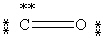
E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
46
Which Lewis structure below obeys the octet rule for every atom in the structure of NBr3 and uses the proper number of valence electrons?
A)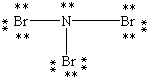
B)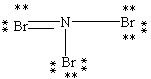
C)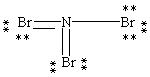
D)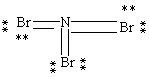
E)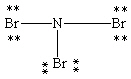
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
47
Which Lewis structure below for (CO3)2 - obeys the octet rule for every atom in the structure and uses the proper number of valence electrons?
A)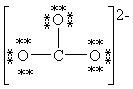
B)
C)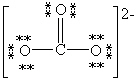
D)
E)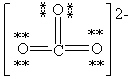
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
48
Which Lewis structure below obeys the octet rule for every atom in the structure of SOCl2 and uses the proper number of valence electrons?
A)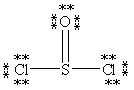
B)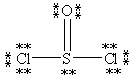
C)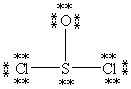
D)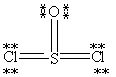
E)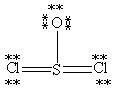
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
49
Which Lewis structure below obeys the octet rule for every atom in the structure of Br2 and uses the proper number of valence electrons?
A)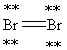
B)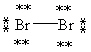
C)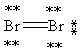
D)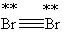
E)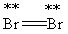
A)
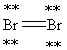
B)
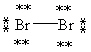
C)
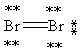
D)
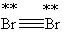
E)
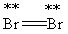

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
50
How many available valence electrons are used in the Lewis structure for the species (NO3)1 - ?
A) 15 e -
B) 16 e -
C) 22 e -
D) 23 e -
E) 24 e -
A) 15 e -
B) 16 e -
C) 22 e -
D) 23 e -
E) 24 e -

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the polyatomic ion, CN1 - . Which Lewis structure below obeys the octet rule for both atoms in the structure and uses the proper number of valence electrons?
A)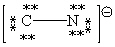
B)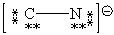
C)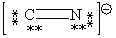
D)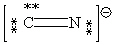
E)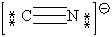
A)
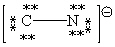
B)
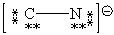
C)
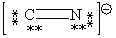
D)
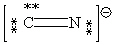
E)
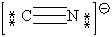

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
52
Which Lewis structure below obeys the octet rule for every atom in the structure of O2 and uses the proper number of valence electrons?
A)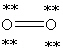
B)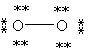
C)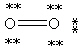
D)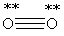
E)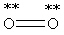
A)
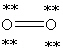
B)

C)

D)
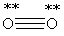
E)
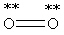

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
53
Which Lewis structure below obeys the octet rule for every atom in the structure of N2 and uses the proper number of valence electrons?
A)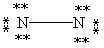
B)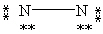
C)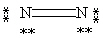
D)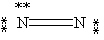
E)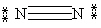
A)
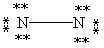
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
54
Which Lewis structure below obeys the octet rule for every atom in the structure of COCl2 and uses the proper number of valence electrons?
A)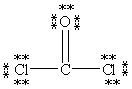
B)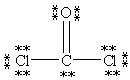
C)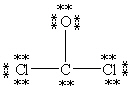
D)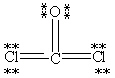
E)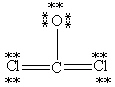
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
55
Which Lewis structure below for hydrazine, N2H4, obeys the octet rule and contains the proper number of valence electrons?
A)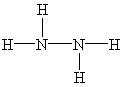
B)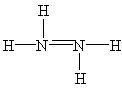
C)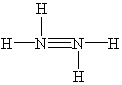
D)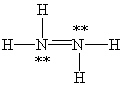
E)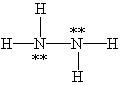
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
56
Which Lewis structure below obeys the octet rule for every atom in the structure of O3 and uses the proper number of valence electrons?
A)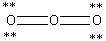
B)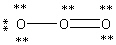
C)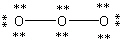
D)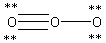
E)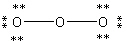
A)
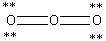
B)
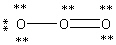
C)

D)
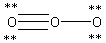
E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
57
Consider the polyatomic ion, ClO1 - . Which Lewis structure below obeys the octet rule for both atoms in the structure and uses the proper number of valence electrons?
A)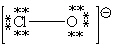
B)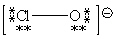
C)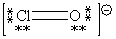
D)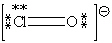
E)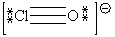
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
58
Consider the polyatomic ion, NO1+. Which Lewis structure below obeys the octet rule for both atoms in the structure and uses the proper number of valence electrons?
A)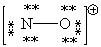
B)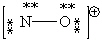
C)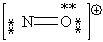
D)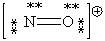
E)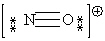
A)
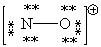
B)
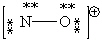
C)
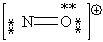
D)
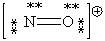
E)
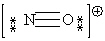

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the polyatomic ion, OH1 - . Which Lewis structure below obeys the octet rule for both atoms in the structure and uses the proper number of valence electrons?
A)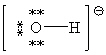
B)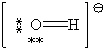
C)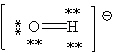
D)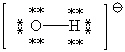
E)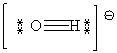
A)
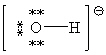
B)
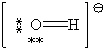
C)
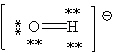
D)

E)
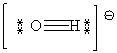

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
60
Which Lewis structure below obeys the octet rule for every atom in the structure of OF2 and uses the proper number of valence electrons?
A)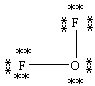
B)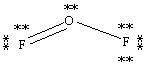
C)
D)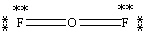
E)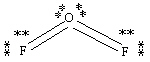
A)

B)

C)

D)
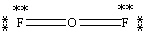
E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
61
Arrange the following bonds in order of increasing polarity . C-F,O-F, Be-F
A) C-F < O-F < Be-F
B) C-F < Be-F < O-F
C) Be-F < O-F < C-F
D) Be-F < C-F < O-F
E) O-F < C-F < Be-F
A) C-F < O-F < Be-F
B) C-F < Be-F < O-F
C) Be-F < O-F < C-F
D) Be-F < C-F < O-F
E) O-F < C-F < Be-F

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
62
Which bond listed below is most polar?
A) C - N
B) Si - N
C) P - N
D) Al - N
E) Ga - N
A) C - N
B) Si - N
C) P - N
D) Al - N
E) Ga - N

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following bonds is most polar?
A) B - Cl
B) Ga - Cl
C) B - S
D) Si - Cl
E) Ga - S
A) B - Cl
B) Ga - Cl
C) B - S
D) Si - Cl
E) Ga - S

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following bonds has the highest polarity?
A) H - O
B) H - F
C) H - Cl
D) H - Br
E) H - I
A) H - O
B) H - F
C) H - Cl
D) H - Br
E) H - I

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
65
Based upon general trends in electronegativity, which polar covalent chemical bond(s) listed below is(are) properly labeled using the vector convention discussed in class?
(Recall that the vector points toward the most electronegative atom in the bond.)
I.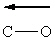
II.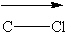
III.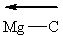
A) II only
B) I and II
C) I and III
D) II and III
E) All of these
(Recall that the vector points toward the most electronegative atom in the bond.)
I.
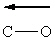
II.

III.
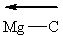
A) II only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the polyatomic ion, NO21 - . Which Lewis structure below obeys the octet rule for both atoms in the structure and uses the proper number of valence electrons?
A)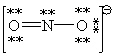
B)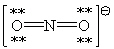
C)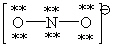
D)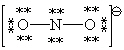
E)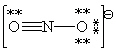
A)
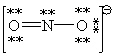
B)
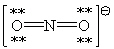
C)
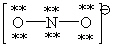
D)
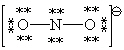
E)
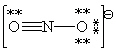

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the polyatomic ion, (SO3)2 - . Which Lewis structure below obeys the octet rule for all atoms in the structure and uses the proper number of valence electrons?
A)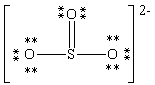
B)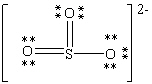
C)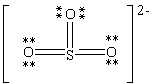
D)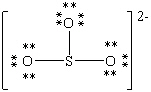
E)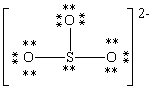
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
68
Which bond would be most polar?
A) O - F
B) Cl - F
C) C - F
D) Al - F
E) B - F
A) O - F
B) Cl - F
C) C - F
D) Al - F
E) B - F

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following elements has the smallest electronegativity?
A) O
B) F
C) Cl
D) Br
E) I
A) O
B) F
C) Cl
D) Br
E) I

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following bonds would be most polar?
A) N - O
B) C - O
C) S - O
D) P - O
E) Si - O
A) N - O
B) C - O
C) S - O
D) P - O
E) Si - O

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
71
Arrange the elements F, P, and Si in order of increasing electronegativity .
A) (least electronegative) F < P < Si (most electronegative)
B) (least electronegative) F < Si < P (most electronegative)
C) (least electronegative) Si < P < F (most electronegative)
D) (least electronegative) Si < F < P (most electronegative)
E) (least electronegative) P < Si < F (most electronegative)
A) (least electronegative) F < P < Si (most electronegative)
B) (least electronegative) F < Si < P (most electronegative)
C) (least electronegative) Si < P < F (most electronegative)
D) (least electronegative) Si < F < P (most electronegative)
E) (least electronegative) P < Si < F (most electronegative)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
72
Consider drawing the Lewis structure for nitrite, NO21 - . Taking into account the charge of this polyatomic anion, how many total valence electrons are present?
A) 17 valence e -
B) 18 valence e -
C) 22 valence e -
D) 23 valence e -
E) 24 valence e -
A) 17 valence e -
B) 18 valence e -
C) 22 valence e -
D) 23 valence e -
E) 24 valence e -

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
73
Arrange the elements As, Cl, and S in order of increasing electronegativity .
A) As < Cl < S
B) As < S < Cl
C) Cl < S < As
D) Cl < As < S
E) S < As < Cl
A) As < Cl < S
B) As < S < Cl
C) Cl < S < As
D) Cl < As < S
E) S < As < Cl

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
74
Arrange the elements O, P, F in order of increasing electronegativity .
A) F < O < P
B) O < F < P
C) F < P < O
D) P < O < F
E) O < P < F
A) F < O < P
B) O < F < P
C) F < P < O
D) P < O < F
E) O < P < F

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
75
Which bond listed below would be most polar?
A) N - N
B) N - P
C) N - C
D) N - As
E) N - Ge
A) N - N
B) N - P
C) N - C
D) N - As
E) N - Ge

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
76
Arrange the elements N, O and Si in order of increasing electronegativity .
A) (least electronegative) N < O < Si (most electronegative)
B) (least electronegative) N < Si < O (most electronegative)
C) (least electronegative) O < N < Si (most electronegative)
D) (least electronegative) O < Si < N (most electronegative)
E) (least electronegative) Si < N < O (most electronegative)
A) (least electronegative) N < O < Si (most electronegative)
B) (least electronegative) N < Si < O (most electronegative)
C) (least electronegative) O < N < Si (most electronegative)
D) (least electronegative) O < Si < N (most electronegative)
E) (least electronegative) Si < N < O (most electronegative)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
77
Arrange the following bonds in order of increasing polarity . F-F, C-F, O-F
A) F-F < C-F < O-F
B) F-F < O-F < C-F
C) O-F < F-F < C-F
D) C-F < F-F < O-F
E) O-F < C-F < F-F
A) F-F < C-F < O-F
B) F-F < O-F < C-F
C) O-F < F-F < C-F
D) C-F < F-F < O-F
E) O-F < C-F < F-F

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the polyatomic ion, (PO3)3 - . Which Lewis structure below obeys the octet rule for both atoms in the structure and uses the proper number of valence electrons?
A)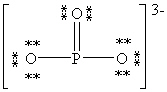
B)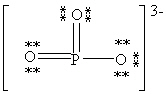
C)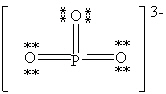
D)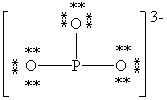
E)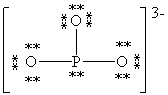
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
79
Arrange the elements K, O, Br in order of increasing electronegativity .
A) K < O < Br
B) Br < O < K
C) K < Br < O
D) O < Br < K
E) O < K < Br
A) K < O < Br
B) Br < O < K
C) K < Br < O
D) O < Br < K
E) O < K < Br

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
80
Based upon electronegativity differences, which of the following bonds is(are) labeled correctly with respect to indicating a bond dipole?
I.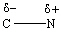
II.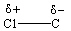
III.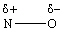
A) I only
B) II only
C) III only
D) I and II
E) II and III
I.

II.

III.

A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck



