Deck 2: Biomolecular Principles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/32
Play
Full screen (f)
Deck 2: Biomolecular Principles
1
The pH of a 0.1 M acetic acid solution is 2.885. What is the dissociation constant of acetic acid?
Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 ![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_77d1_9432_b53a2bd133bf_SM1184_00.jpg) The acetic acid is dissociated as acetate ion and hydrogen ion.
The acetic acid is dissociated as acetate ion and hydrogen ion. ![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_77d2_9432_152868d77721_SM1184_11.jpg) The dissociation constant of acetic acid is calculated as follows:
The dissociation constant of acetic acid is calculated as follows: ![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_77d3_9432_5d17ada2f379_SM1184_11.jpg)
![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_77d4_9432_8f20ae3e210f_SM1184_11.jpg) Therefore, the dissociation constant of acetic acid is
Therefore, the dissociation constant of acetic acid is ![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_9ee5_9432_b543b8bb7728_SM1184_11.jpg)
![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_77d1_9432_b53a2bd133bf_SM1184_00.jpg) The acetic acid is dissociated as acetate ion and hydrogen ion.
The acetic acid is dissociated as acetate ion and hydrogen ion. ![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_77d2_9432_152868d77721_SM1184_11.jpg) The dissociation constant of acetic acid is calculated as follows:
The dissociation constant of acetic acid is calculated as follows: ![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_77d3_9432_5d17ada2f379_SM1184_11.jpg)
![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_77d4_9432_8f20ae3e210f_SM1184_11.jpg) Therefore, the dissociation constant of acetic acid is
Therefore, the dissociation constant of acetic acid is ![Given [H + ] = 0.1 M acetic acid solution have pH = 2.885 The acetic acid is dissociated as acetate ion and hydrogen ion. The dissociation constant of acetic acid is calculated as follows: Therefore, the dissociation constant of acetic acid is](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ad_9ee5_9432_b543b8bb7728_SM1184_11.jpg)
2
A solution initially contains 42 mM formic acid (HCHO2, pKA = 3.76).
a. Formic acid is a weak acid and partially ionizes in water. Write a balanced chemical reaction for its dissociation.
b. Determine the conjugate base and H+ concentration at equilibrium.
c. Calculate the percentage of ionization.
a. Formic acid is a weak acid and partially ionizes in water. Write a balanced chemical reaction for its dissociation.
b. Determine the conjugate base and H+ concentration at equilibrium.
c. Calculate the percentage of ionization.
a. The balanced chemical reaction for the dissociation formic acid is as follows: 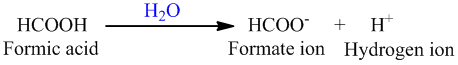 b. The conjugate base in the reaction after the dissociation is HCOO - or the formate ion. The H + ion concentration at equilibrium will be equal to the concentration of formic acid.
b. The conjugate base in the reaction after the dissociation is HCOO - or the formate ion. The H + ion concentration at equilibrium will be equal to the concentration of formic acid.
c. The percentage of ionization is given by the equation: Initial concentration = 42 mM,
Initial concentration = 42 mM,
The amount dissociated = 21 mM
(Formic acid partially dissociates in water)
Therefore,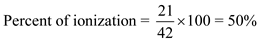 Therefore, the percentage of ionization = 50%.
Therefore, the percentage of ionization = 50%.
 b. The conjugate base in the reaction after the dissociation is HCOO - or the formate ion. The H + ion concentration at equilibrium will be equal to the concentration of formic acid.
b. The conjugate base in the reaction after the dissociation is HCOO - or the formate ion. The H + ion concentration at equilibrium will be equal to the concentration of formic acid.c. The percentage of ionization is given by the equation:
 Initial concentration = 42 mM,
Initial concentration = 42 mM, The amount dissociated = 21 mM
(Formic acid partially dissociates in water)
Therefore,
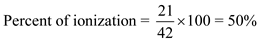 Therefore, the percentage of ionization = 50%.
Therefore, the percentage of ionization = 50%. 3
How does hyperventilation-that is, very rapid deep breathing-disturb the HCO−3 /H2CO3 equilibrium? Does it result in acidosis or alkalosis?
The bicarbonate buffering system maintains the body in constant stage near neutral pH of blood. The blood containing bicarbonate buffering system, which has a mixture of carbonic acid (H 2 CO 3 ) and sodium bicarbonate (NaHCO 3 ) acts as buffer to change the extremities in pH. The presence of carbonic acid and bicarbonate equilibrium in a solution is apt to neutralize the solution.
To maintain the pH of 7.4, the body maintains a ratio of [HCO 3 -/H 2 CO 3 ] near 20. The excretion of HCO 3 - is controlled by the kidneys and that of H 2 CO 3 is controlled by lungs through respiration to increase or decrease CO 2. Hyperventilation that is very rapid deep breathing disturbs the HCO 3 - /H 2 CO 3 equilibrium and elevates the blood pH. This condition leads to alkalosis. Thus, this disorder is also called as respiratory alkalosis.
To maintain the pH of 7.4, the body maintains a ratio of [HCO 3 -/H 2 CO 3 ] near 20. The excretion of HCO 3 - is controlled by the kidneys and that of H 2 CO 3 is controlled by lungs through respiration to increase or decrease CO 2. Hyperventilation that is very rapid deep breathing disturbs the HCO 3 - /H 2 CO 3 equilibrium and elevates the blood pH. This condition leads to alkalosis. Thus, this disorder is also called as respiratory alkalosis.
4
For the dissociation reaction of a weak acid shown below, begin with defining the Ka and show all the steps for the derivation of the Henderson-Hasselbalch equation. 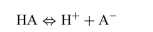 The Henderson-Hasselbalch equation for the blood bicarbonate system is shown as follows:
The Henderson-Hasselbalch equation for the blood bicarbonate system is shown as follows: 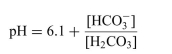
a. Calculate the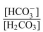 ratio for a blood pH of 5.8.
ratio for a blood pH of 5.8.
b. Is this patient experiencing acidosis or alkalosis? Why?
c. What can the body due to restore the blood pH to normal?
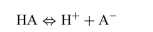 The Henderson-Hasselbalch equation for the blood bicarbonate system is shown as follows:
The Henderson-Hasselbalch equation for the blood bicarbonate system is shown as follows: 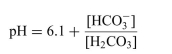
a. Calculate the
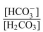 ratio for a blood pH of 5.8.
ratio for a blood pH of 5.8. b. Is this patient experiencing acidosis or alkalosis? Why?
c. What can the body due to restore the blood pH to normal?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
5
Write the condensation reactions involved in the synthesis of a disaccha- ride from two monosaccharides, a dipeptide from two amino acids, and a dinucleotide from two nucleotides.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
6
What is the pH of a buffer solution that is 0.20 M proprionic acid 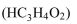 and 0.1 M sodium proprionate
and 0.1 M sodium proprionate 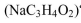 The KA of proprionic acid is
The KA of proprionic acid is 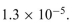
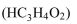 and 0.1 M sodium proprionate
and 0.1 M sodium proprionate 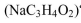 The KA of proprionic acid is
The KA of proprionic acid is 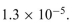

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
7
Describe the properties of acids and bases. It might be helpful to look in a Chemistry book, to find information beyond that available in this chapter.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
8
Do some research in the library or on the internet, using reliable sources. Cystic fibrosis is a genetic disease. What is the defect in cystic fibrosis patients, and how does that defect manifest into the symptoms for the disease?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
9
For each of the following compounds, classify it as an acid or a base: a) NH3, b) H3PO4, c) LiOH, d) HCOOH (formic acid), e) H2SO4, f) HF, g) Ba(OH)2.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
10
Carbohydrates in foods are a source of energy. The combustion of glucose ![Carbohydrates in foods are a source of energy. The combustion of glucose is: a. Calculate the standard enthalpy of the reaction. HINT: Use heats of formation from Appendix B, Table B.2. b. Is this an exothermic or endothermic process? How much heat (kcal) is generated for each gram of glucose that is burned? c. Calculate the value of is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ae_141c_9432_791a9deb07f3_SM1184_11.jpg) is:
is: ![Carbohydrates in foods are a source of energy. The combustion of glucose is: a. Calculate the standard enthalpy of the reaction. HINT: Use heats of formation from Appendix B, Table B.2. b. Is this an exothermic or endothermic process? How much heat (kcal) is generated for each gram of glucose that is burned? c. Calculate the value of is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ae_141d_9432_bddb613b064b_SM1184_11.jpg)
a. Calculate the standard enthalpy of the reaction. HINT: Use heats of formation from Appendix B, Table B.2.
b. Is this an exothermic or endothermic process? How much heat (kcal) is generated for each gram of glucose that is burned?
c. Calculate the value of![Carbohydrates in foods are a source of energy. The combustion of glucose is: a. Calculate the standard enthalpy of the reaction. HINT: Use heats of formation from Appendix B, Table B.2. b. Is this an exothermic or endothermic process? How much heat (kcal) is generated for each gram of glucose that is burned? c. Calculate the value of is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ae_3b2e_9432_75afbaa71da7_SM1184_11.jpg) is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]
is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]
![Carbohydrates in foods are a source of energy. The combustion of glucose is: a. Calculate the standard enthalpy of the reaction. HINT: Use heats of formation from Appendix B, Table B.2. b. Is this an exothermic or endothermic process? How much heat (kcal) is generated for each gram of glucose that is burned? c. Calculate the value of is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ae_141c_9432_791a9deb07f3_SM1184_11.jpg) is:
is: ![Carbohydrates in foods are a source of energy. The combustion of glucose is: a. Calculate the standard enthalpy of the reaction. HINT: Use heats of formation from Appendix B, Table B.2. b. Is this an exothermic or endothermic process? How much heat (kcal) is generated for each gram of glucose that is burned? c. Calculate the value of is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ae_141d_9432_bddb613b064b_SM1184_11.jpg)
a. Calculate the standard enthalpy of the reaction. HINT: Use heats of formation from Appendix B, Table B.2.
b. Is this an exothermic or endothermic process? How much heat (kcal) is generated for each gram of glucose that is burned?
c. Calculate the value of
![Carbohydrates in foods are a source of energy. The combustion of glucose is: a. Calculate the standard enthalpy of the reaction. HINT: Use heats of formation from Appendix B, Table B.2. b. Is this an exothermic or endothermic process? How much heat (kcal) is generated for each gram of glucose that is burned? c. Calculate the value of is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]](https://d2lvgg3v3hfg70.cloudfront.net/SM1184/11ec7385_02ae_3b2e_9432_75afbaa71da7_SM1184_11.jpg) is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]
is 212 J/(K-mol). Is this a favorable reaction? [Note: 1 cal = 4.184 J]
Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
11
Why are polar molecules hydrophilic and nonpolar molecules hydro- phobic?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
12
A U-tube apparatus (as in Box 2.6) is separated by a membrane permeable to water, but not to sodium chloride (NaCl). NaCl (8 g) is dissolved in 0.5 L of water and placed on one side of a semipermeable membrane with pure water on the other side of the membrane. Draw a diagram of the beaker. Which direction will the water flow? If the temperature of the water is constant at 25◦C, what is the osmotic pressure? If compartment A and B begin with equal volumes, what will be the difference in the height of the fluid columns at equilibrium?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
13
For the following substances, draw the chemical structure and determine whether the substance is polar or nonpolar. If it is polar, indicate the partial negative and positive charges on the appropriate atoms.
a. Carbon dioxid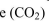
b. Carbon tetrachloride (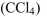
c. Hydrochloric acid (HCl)
d. Ammonia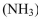
e. Oxygen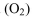
a. Carbon dioxid
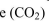
b. Carbon tetrachloride (
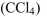
c. Hydrochloric acid (HCl)
d. Ammonia
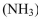
e. Oxygen
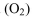

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
14
The first step in glycolysis (breakdown of sugar) is to convert glucose to glucose-6-phosphate. Calculate the equilibrium constant for the reaction at 25◦C. Is this reaction favorable or not? If it is not favorable, what can drive the reaction to proceed as written? 


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
15
If hydrogen bonds are much weaker than covalent bonds, why do you think hydrogen bonds are used to hold biomolecules together?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
16
In vitro experiments are conducted at pH = 7.4 to simulate physiological conditions. A phosphate buffer system is often used. 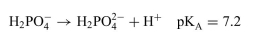
a. What must be the ratio of the concentrations of HPO24− to H2PO−4 ions?
b. What mass of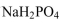 must be added to 500.0 mL of 0.10 M Na2HPO4 (aq) in the preparation of the buffered solution?
must be added to 500.0 mL of 0.10 M Na2HPO4 (aq) in the preparation of the buffered solution?
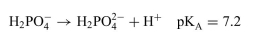
a. What must be the ratio of the concentrations of HPO24− to H2PO−4 ions?
b. What mass of
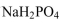 must be added to 500.0 mL of 0.10 M Na2HPO4 (aq) in the preparation of the buffered solution?
must be added to 500.0 mL of 0.10 M Na2HPO4 (aq) in the preparation of the buffered solution?
Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
17
Tyrosine, serine, and threonine are amino acids, which can be modified by phosphorylation (addition of phosphate group). As you will see, this is an important mechanism for turning enzymes on or off. (a) Find the chemical structures for tyrosine, serine, and threonine and draw them (see Appen- dix B, Table B.1). For each of the structures, (b) identify each functional group in the molecule, and (c) determine whether the molecule can undergo hydrogen bonding. Mark the partial charges on the appropriate atoms.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
18
Estimate the flux (mg/cm2/s) by diffusion of a steroid through a lipid bilayer membrane. Assume the diffusion coefficient for steroid in the lipid bilayer is 10−6 cm2/s, and that the concentration is 1 ng/mL on the outside of the membrane and 0 on the inside.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
19
Does entropy increase or decrease during a polymerization reaction? Why?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
20
For the membrane of thickness 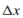 shown in the Box 2.5:
shown in the Box 2.5:
a. Draw a graph of the concentration of solute as a function of x at steady state.
b. Estimate the concentration profiles that you expect during the approach to steady state. That is, assume that the membrane is initially saturated with solute at concentration, C2, and then the concentration on the left boundary (at x = 0) is suddenly increased to C1. Sketch the concentration profile immediately after the increase to C1. Sketch the concentration profile a little later, but before steady state is achieved.
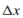 shown in the Box 2.5:
shown in the Box 2.5: a. Draw a graph of the concentration of solute as a function of x at steady state.
b. Estimate the concentration profiles that you expect during the approach to steady state. That is, assume that the membrane is initially saturated with solute at concentration, C2, and then the concentration on the left boundary (at x = 0) is suddenly increased to C1. Sketch the concentration profile immediately after the increase to C1. Sketch the concentration profile a little later, but before steady state is achieved.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
21
Identify the acid and conjugate base in each reaction. Calculate the pKa for each acid. List them in order from the strongest to weakest acid. The acid-ionization constants, Ka,at25◦C are listed for each.
a.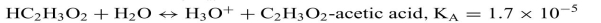
b.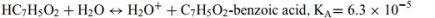
c.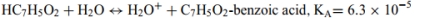
d.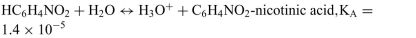
a.
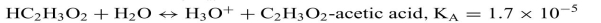
b.
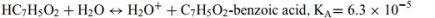
c.
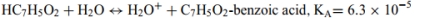
d.
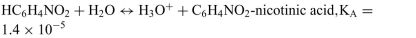

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
22
A solution of 1 M glucose is separated by a selectively permeable membrane from a solution of 0.2 M fructose and 0.7 M sucrose. The membrane is not permeable to any of the sugar molecules. Indicate which side of the membrane is initially hypertonic, which is hypotonic, and the direction of water movement.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
23
Explain the difference between passive and active transport. Why is active transport necessary for some ions?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
24
Consider a U-shaped tube (as illustrated in Box 2.6) in which the arms of the U-tube are separated by a membrane that is permeable to water and glucose but not sucrose. The left side (side A) is filled with a solution of 2.0 M sucrose and 1.0 M glucose. The right side (side B) is filled with 1.0 M sucrose and 2.0 M glucose.
a. What changes would you observe, as the system moves toward equilib- rium?
b. During the period from initial filling to equilibrium, which molecule(s) will show net movement through the membrane?
a. What changes would you observe, as the system moves toward equilib- rium?
b. During the period from initial filling to equilibrium, which molecule(s) will show net movement through the membrane?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
25
Calculate the [H+] of stomach acid and blood. Which has a higher [H+]? What generalization can you make regarding the relationship between [H+] and pH?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
26
One of the components in the head of "strike-anywhere" matches is tetra- phosphorus trisulfide, P4S3. The combustion is shown below.
a. Calculate the standard enthalpy of the reaction.
b. Draw a graphical representation of the standard enthalpy change for this reaction.
c. Is this an exothermic or endothermic process? Explain your answer.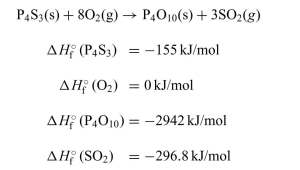
a. Calculate the standard enthalpy of the reaction.
b. Draw a graphical representation of the standard enthalpy change for this reaction.
c. Is this an exothermic or endothermic process? Explain your answer.
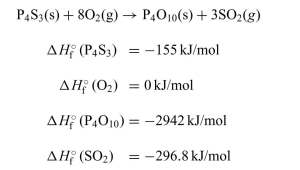

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
27
Normal saline solution (0.9% NaCl by mass) is used for intravenous admin- istration or for lubrication of dry eyes. Do you think that this solution is isotonic, hypertonic, or hypotonic compared to the body fluids? Why?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
28
The decomposition of calcium carbonate is shown below along with the standard enthalpy and entropy values.
a. Calculate the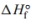 for the reaction.
for the reaction.
b. Calculate the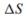 for the reaction.
for the reaction.
c. What is the standard Gibb's free-energy change expression for the reac- tion?
d. Is the reaction spontaneous at 25◦C? Is the reaction spontaneous at 1000◦C? Explain your answers.
e. Calculate the equilibrium constant at 25◦C and 1000◦C.
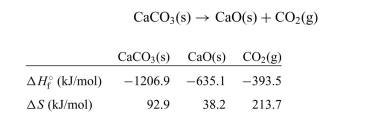
a. Calculate the
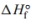 for the reaction.
for the reaction. b. Calculate the
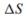 for the reaction.
for the reaction. c. What is the standard Gibb's free-energy change expression for the reac- tion?
d. Is the reaction spontaneous at 25◦C? Is the reaction spontaneous at 1000◦C? Explain your answers.
e. Calculate the equilibrium constant at 25◦C and 1000◦C.


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
29
A solution contains 0.45 M hydrofluoric acid 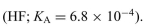 Write the dissociation reaction. Determine the degree of ionization and the pH of the solution.
Write the dissociation reaction. Determine the degree of ionization and the pH of the solution.
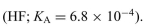 Write the dissociation reaction. Determine the degree of ionization and the pH of the solution.
Write the dissociation reaction. Determine the degree of ionization and the pH of the solution.
Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
30
The reaction in which urea is formed from NH3 and CO2 is shown below. The standard free-energy change 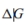 at 25◦Cis−13.6 kJ/mol.
at 25◦Cis−13.6 kJ/mol. 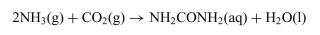
a. Write an expression for the equilibrium constant, K, in terms of the molar concentrations of the reactants and products.
b. Write an expression for the equilibrium constant, K, as a function of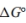 and temperature.
and temperature.
c. Determine the value of the equilibrium constant, K, for this reaction at 25◦C.
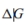 at 25◦Cis−13.6 kJ/mol.
at 25◦Cis−13.6 kJ/mol. 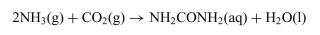
a. Write an expression for the equilibrium constant, K, in terms of the molar concentrations of the reactants and products.
b. Write an expression for the equilibrium constant, K, as a function of
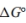 and temperature.
and temperature. c. Determine the value of the equilibrium constant, K, for this reaction at 25◦C.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
31
If you are on a deserted island, why must you find water from a stream or well rather than drink the seawater? Explain your answer in terms of osmotic pressure.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
32
Acetic acid, CH3COOH, is a typical weak acid. It is an ingredient in vinegar.
a. Acetic acid partially ionizes in water. Write a balanced chemical reaction for the dissociation of acetic acid into its conjugate base and hydrogen ion.
b. Write an expression for the equilibrium constant for acetic acid.
c. The equilibrium concentrations are [CH3COOH] = 0.15M, [CH3COOH] = 0.15M, and [CH3COO-] = 1.63mM. What is the equi- librium constant of ionization, KA?
d. Calculate the pKA of acetic acid.
a. Acetic acid partially ionizes in water. Write a balanced chemical reaction for the dissociation of acetic acid into its conjugate base and hydrogen ion.
b. Write an expression for the equilibrium constant for acetic acid.
c. The equilibrium concentrations are [CH3COOH] = 0.15M, [CH3COOH] = 0.15M, and [CH3COO-] = 1.63mM. What is the equi- librium constant of ionization, KA?
d. Calculate the pKA of acetic acid.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck



