Deck 15: Acid-Base Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 15: Acid-Base Equilibria
1
Which of the following is a Lewis acid?
A) Fe2+
B) BH3
C) NH3
D) a and b
E) a and c
A) Fe2+
B) BH3
C) NH3
D) a and b
E) a and c
D
2
The value of Kw increases slightly over the temperature range 0 to 60°C. Compared to its value at 0°C, the pH of water at room temperature will be:
A) higher
B) lower
C) the same
D) not enough data to answer the question
A) higher
B) lower
C) the same
D) not enough data to answer the question
B
3
The value of Kw increases slightly over the temperature range 0 to 60°C. Compared to its value at 0°C, the pH of water at room temperature will be:
A) higher
B) lower
C) the same
D) not enough data to answer the question
A) higher
B) lower
C) the same
D) not enough data to answer the question
B
4
Which of the following is the strongest acid:
A) NH3
B) PH3
C) Both are equally strong
D) Not enough data to answer the question
A) NH3
B) PH3
C) Both are equally strong
D) Not enough data to answer the question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
In pure water:
A) There are no detectible ions in solution
B) There are detectible ions in solution
C) H3O+ and OH- ions are more prevalent than H2O molecules
D) a and c
E) b and c
A) There are no detectible ions in solution
B) There are detectible ions in solution
C) H3O+ and OH- ions are more prevalent than H2O molecules
D) a and c
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
Ka = 6.6×10-4 for the reaction:
HF + H2O H3O+ + F-
H3O+ + F-
What is Kb for the reaction?
F- + H2O HF + OH-
HF + OH-
A) 6.6×10-4
B) 1.6×104
C) 6.6×10-18
D) 1.5×10-11
HF + H2O
 H3O+ + F-
H3O+ + F-What is Kb for the reaction?
F- + H2O
 HF + OH-
HF + OH-A) 6.6×10-4
B) 1.6×104
C) 6.6×10-18
D) 1.5×10-11

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
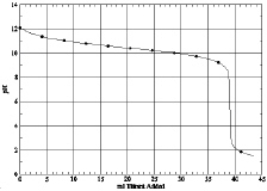
Which of the following is the titration curve of strong acid being titrated by a strong base
A)
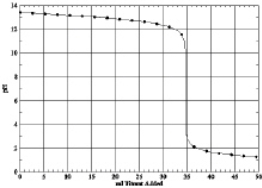
B)
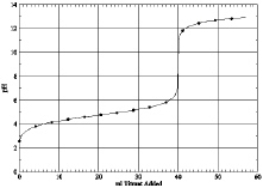
C)
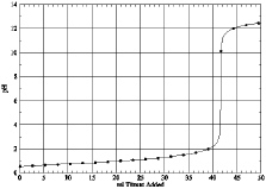
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the titration curve of weak base being titrated by a strong acid
A)
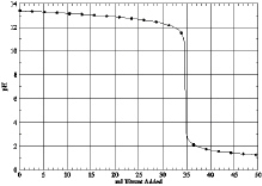
B)
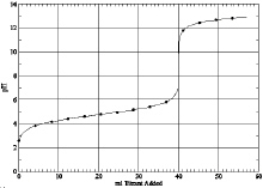
C)
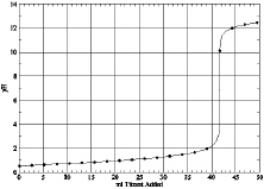
D)
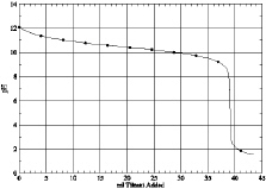
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 15-1
The following question(s) pertain to the titration of 50.0 ml of HNO3 with NaOH as depicted in the plot below. Refer to Exhibit 15-1. Estimate the initial concentration of Nitric Acid
Refer to Exhibit 15-1. Estimate the initial concentration of Nitric Acid
A) 0.50 M
B) 0.32 M
C) 1.0 M
D) 15.0 M
The following question(s) pertain to the titration of 50.0 ml of HNO3 with NaOH as depicted in the plot below.
 Refer to Exhibit 15-1. Estimate the initial concentration of Nitric Acid
Refer to Exhibit 15-1. Estimate the initial concentration of Nitric AcidA) 0.50 M
B) 0.32 M
C) 1.0 M
D) 15.0 M

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 15-1
The following question(s) pertain to the titration of 50.0 ml of HNO3 with NaOH as depicted in the plot below. Refer to Exhibit 15-1. Estimate the initial concentration of Sodium Hydroxide
Refer to Exhibit 15-1. Estimate the initial concentration of Sodium Hydroxide
A) 0.38 M
B) 0.42 M
C) 0.60 M
D) 0.55 M
The following question(s) pertain to the titration of 50.0 ml of HNO3 with NaOH as depicted in the plot below.
 Refer to Exhibit 15-1. Estimate the initial concentration of Sodium Hydroxide
Refer to Exhibit 15-1. Estimate the initial concentration of Sodium HydroxideA) 0.38 M
B) 0.42 M
C) 0.60 M
D) 0.55 M

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
A student has 100.0 ml of 0.65 M HCl. What is the pH of this solution?
A) 1.39
B) 0.187
C) 6.50
D) 13.8
E) None of the above
A) 1.39
B) 0.187
C) 6.50
D) 13.8
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
A student has 50.0 ml of 0.30 M NaOH. What is the pH of this solution?
A) 1.82
B) 11.00
C) 0.523
D) 2.0
E) None of the above
A) 1.82
B) 11.00
C) 0.523
D) 2.0
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
A student mixes 60.0 ml of 0.30 M NaOH with 20.0 ml of 0.90 M HCl. What is the pH of the resulting solution?
A) 0
B) 14
C) 7
D) 1.27
E) None of the above
A) 0
B) 14
C) 7
D) 1.27
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Based on the plot below, estimate the value of Ka for the weak acid. 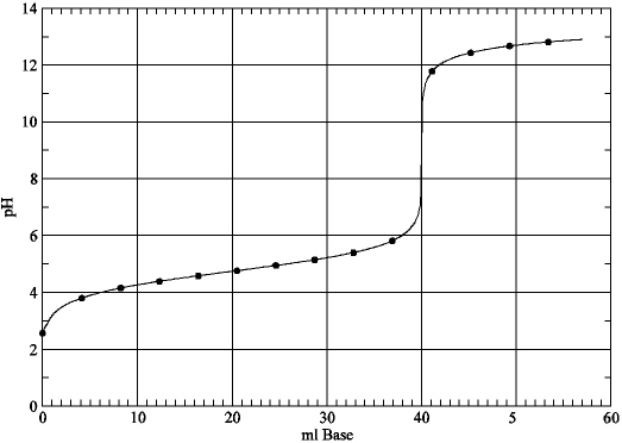
A) 4.7
B) 2.0×10-5
C) 2.6
D) 2.5×10-3
E) None of the above

A) 4.7
B) 2.0×10-5
C) 2.6
D) 2.5×10-3
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 15-2
The following question(s) pertain to the sequence of events below:
Part A: A solution initially only containing 500.0 ml of 1.00 M Formic Acid.
Part B: 800.0 ml of 0.500 M NaOH is added to the solution from Part A.
Part C: An additional 250.0 ml of the 0.500 M NaOH is added to the resulting solution from Part B.
Refer to Exhibit 15-2,Part A. What is the pH of the solution?
A) 3.75
B) 1.83
C) 0
D) None of the above
The following question(s) pertain to the sequence of events below:
Part A: A solution initially only containing 500.0 ml of 1.00 M Formic Acid.
Part B: 800.0 ml of 0.500 M NaOH is added to the solution from Part A.
Part C: An additional 250.0 ml of the 0.500 M NaOH is added to the resulting solution from Part B.
Refer to Exhibit 15-2,Part A. What is the pH of the solution?
A) 3.75
B) 1.83
C) 0
D) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 15-2 The following question(s) pertain to the sequence of events below:
Part A: A solution initially only containing 500.0 ml of 1.00 M Formic Acid.
Part B: 800.0 ml of 0.500 M NaOH is added to the solution from Part A.
Part C: An additional 250.0 ml of the 0.500 M NaOH is added to the resulting solution from Part B.
Refer to Exhibit 15-2, Part B. What is the resulting pH?
A) 1.11
B) 4.35
C) 3.15
D) None of the above
Part A: A solution initially only containing 500.0 ml of 1.00 M Formic Acid.
Part B: 800.0 ml of 0.500 M NaOH is added to the solution from Part A.
Part C: An additional 250.0 ml of the 0.500 M NaOH is added to the resulting solution from Part B.
Refer to Exhibit 15-2, Part B. What is the resulting pH?
A) 1.11
B) 4.35
C) 3.15
D) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
Exhibit 15-2 The following question(s) pertain to the sequence of events below:
Part A: A solution initially only containing 500.0 ml of 1.00 M Formic Acid.
Part B: 800.0 ml of 0.500 M NaOH is added to the solution from Part A.
Part C: An additional 250.0 ml of the 0.500 M NaOH is added to the resulting solution from Part B.
Refer to Exhibit 15-2, Part C. What is the new pH?
A) 3.05
B) 10.94
C) 12.20
D) 13.33
Part A: A solution initially only containing 500.0 ml of 1.00 M Formic Acid.
Part B: 800.0 ml of 0.500 M NaOH is added to the solution from Part A.
Part C: An additional 250.0 ml of the 0.500 M NaOH is added to the resulting solution from Part B.
Refer to Exhibit 15-2, Part C. What is the new pH?
A) 3.05
B) 10.94
C) 12.20
D) 13.33

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
A student dissolves 30.00 grams of sodium acetate in exactly one liter of water. What will be the pH of the resulting solution?
A) 9.68
B) 9.24
C) 9.16
D) 4.84
A) 9.68
B) 9.24
C) 9.16
D) 4.84

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
What will have the higher pOH, a 1.0 M solution of sodium acetate of a 1.0 M solution of sodium benzoate?
A) Sodium benzoate
B) Sodium acetate
C) Both pH values will be near 7
D) None of the above
A) Sodium benzoate
B) Sodium acetate
C) Both pH values will be near 7
D) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
A student wants to make a pH 4.0 buffer solution. She decides to use a buffer made from formic acid and sodium formate (NaCOOH). She adds 50.0 grams of sodium formate to a 1 L volumetric flask. How many mL of 3.0 M formic acid should she add to the 1 L volumetric flask so that when the flask is diluted to the mark the pH will be 4.00?
A) 13.8 ml
B) 138 ml
C) 72.6 ml
D) 726 ml
E) None of the above
A) 13.8 ml
B) 138 ml
C) 72.6 ml
D) 726 ml
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
Solutions containing the H2PO4- and HPO42- ions should respectively
A) both be acidic
B) both be basic
C) be acidic and basic
D) be basic and acidic
A) both be acidic
B) both be basic
C) be acidic and basic
D) be basic and acidic

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 15-3
The following question(s) pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
Refer to Exhibit 15-3. When adding strong acid to this solution:
A) the benzoic acid concentration should rise and the benzoate concentration should fall
B) the benzoic acid concentration should fall and the benzoate concentration should rise
C) both benzoic acid and sodium benzoate concentrations should fall
D) the concentration of all ions should remain roughly constant
E) None of the above
The following question(s) pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
Refer to Exhibit 15-3. When adding strong acid to this solution:
A) the benzoic acid concentration should rise and the benzoate concentration should fall
B) the benzoic acid concentration should fall and the benzoate concentration should rise
C) both benzoic acid and sodium benzoate concentrations should fall
D) the concentration of all ions should remain roughly constant
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 15-3
The following question(s) pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
Refer to Exhibit 15-3. What is pH of this solution?
A) 4.04
B) 4.19
C) 4.33
D) 9.81
E) None of the above
The following question(s) pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
Refer to Exhibit 15-3. What is pH of this solution?
A) 4.04
B) 4.19
C) 4.33
D) 9.81
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 15-3
The following question(s) pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
Refer to Exhibit 15-3. Calculate the pH after the addition of 500 ml of 1.00 M HCl.
A) 0.175
B) 0.876
C) 3.48
D) 4.89
E) None of the above
The following question(s) pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
Refer to Exhibit 15-3. Calculate the pH after the addition of 500 ml of 1.00 M HCl.
A) 0.175
B) 0.876
C) 3.48
D) 4.89
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Exhibit 15-3
The following question(s) pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
Refer to Exhibit 15-3. Calculate the pH after the addition of an additional 250 ml of 1.00 M HCl.
A) 0.339
B) 1.54
C) 2.98
D) 5.38
E) None of the above
The following question(s) pertain to a solution made by combining benzoic acid and sodium benzoate. The final volume of the solution is 1.00 L and the initial concentrations of benzoic acid and sodium benzoate are 0.50 and 0.70 respectively.
Refer to Exhibit 15-3. Calculate the pH after the addition of an additional 250 ml of 1.00 M HCl.
A) 0.339
B) 1.54
C) 2.98
D) 5.38
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
Exhibit 15-4
The following question(s) pertain to a solution prepared by combining 1.0 mole of ammonia and 50.0 grams of ammonium nitrate in enough water to give a final volume of 2.00 liters.
Refer to Exhibit 15-4. When adding strong acid to this solution:
A) the ammonia concentration should rise
B) the ammonium ion concentration should fall
C) both a and b
D) None of the above
The following question(s) pertain to a solution prepared by combining 1.0 mole of ammonia and 50.0 grams of ammonium nitrate in enough water to give a final volume of 2.00 liters.
Refer to Exhibit 15-4. When adding strong acid to this solution:
A) the ammonia concentration should rise
B) the ammonium ion concentration should fall
C) both a and b
D) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 15-4
The following question(s) pertain to a solution prepared by combining 1.0 mole of ammonia and 50.0 grams of ammonium nitrate in enough water to give a final volume of 2.00 liters.
Refer to Exhibit 15-4. What is pH of this solution?
A) 9.46
B) 9.55
C) 9.04
D) 4.95
E) None of the above
The following question(s) pertain to a solution prepared by combining 1.0 mole of ammonia and 50.0 grams of ammonium nitrate in enough water to give a final volume of 2.00 liters.
Refer to Exhibit 15-4. What is pH of this solution?
A) 9.46
B) 9.55
C) 9.04
D) 4.95
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
Exhibit 15-4
The following question(s) pertain to a solution prepared by combining 1.0 mole of ammonia and 50.0 grams of ammonium nitrate in enough water to give a final volume of 2.00 liters.
Refer to Exhibit 15-4. Calculate the pH after the addition of 500 ml of 1.00 M HCl.
A) 4.40
B) 5.10
C) 8.90
D) 9.04
E) None of the above
The following question(s) pertain to a solution prepared by combining 1.0 mole of ammonia and 50.0 grams of ammonium nitrate in enough water to give a final volume of 2.00 liters.
Refer to Exhibit 15-4. Calculate the pH after the addition of 500 ml of 1.00 M HCl.
A) 4.40
B) 5.10
C) 8.90
D) 9.04
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 15-4
The following question(s) pertain to a solution prepared by combining 1.0 mole of ammonia and 50.0 grams of ammonium nitrate in enough water to give a final volume of 2.00 liters.
Refer to Exhibit 15-4. Calculate the pH after the addition of an additional 250 ml of 1.00 M HCl.
A) 0.266
B) 2.51
C) 4.76
D) 7.75
E) None of the above
The following question(s) pertain to a solution prepared by combining 1.0 mole of ammonia and 50.0 grams of ammonium nitrate in enough water to give a final volume of 2.00 liters.
Refer to Exhibit 15-4. Calculate the pH after the addition of an additional 250 ml of 1.00 M HCl.
A) 0.266
B) 2.51
C) 4.76
D) 7.75
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
Predict whether a 0.500 M aqueous solution of sodium hydrogen sulfite (NaHSO3) should be acidic, basic, or neutral.
A) acidic
B) neutral
C) basic
A) acidic
B) neutral
C) basic

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
A solution is prepared by mixing hydrazoic acid (HN3) and NaN3 and then allowed to come to equilibrium. Upon addition of sulfuric acid
A) more NaN3 will be formed
B) More HN3 will be formed
C) More N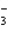 will be formed
will be formed
D) Both A and B
E) Impossible to answer without knowing equilibrium concentrations
A) more NaN3 will be formed
B) More HN3 will be formed
C) More N
 will be formed
will be formedD) Both A and B
E) Impossible to answer without knowing equilibrium concentrations

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
You have at your disposal 100.00 mL of 0.650 M HCl and 500.00 mL of 0.300 M NaOH. How many mL of the base have to be added to the full volume of the acid to reach a pH of 1.90?
A) 10.2 mL
B) 20.4 mL
C) 43.3 mL
D) 204 mL
E) 433 mL
A) 10.2 mL
B) 20.4 mL
C) 43.3 mL
D) 204 mL
E) 433 mL

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following titration curves schematically represents a diprotic acid being titrated by a strong base?
A)
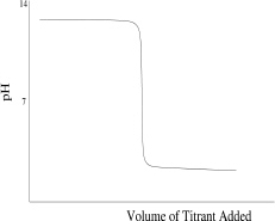
B)
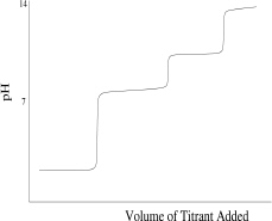
C)
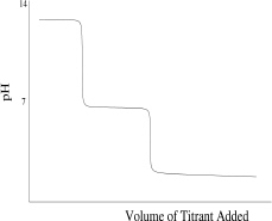
D)
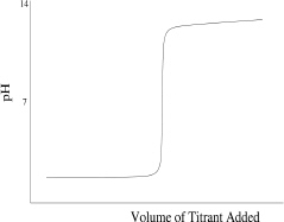
E) None of the above
A)

B)

C)

D)

E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck



