Deck 2: The Chemistry of Microbiology
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/76
Play
Full screen (f)
Deck 2: The Chemistry of Microbiology
1
Which of the following is a property of water?
A) it has a high capacity for heat.
B) it is not a common reactant in metabolic reactions.
C) It is not a good solvent.
D) it is liquid in a very narrow temperature range.
E) it is a nonpolar molecule.
A) it has a high capacity for heat.
B) it is not a common reactant in metabolic reactions.
C) It is not a good solvent.
D) it is liquid in a very narrow temperature range.
E) it is a nonpolar molecule.
A
2
Unstable isotopes can be useful
A) catalysts.
B) in medical diagnosis.
C) in vitamins.
D) in the formation of hydrogen bonds.
E) as buffers.
A) catalysts.
B) in medical diagnosis.
C) in vitamins.
D) in the formation of hydrogen bonds.
E) as buffers.
B
3
Organisms use carbohydrates in all of the following ways EXCEPT
A) as a component of cell walls.
B) as a long-term energy source.
C) as a short-term energy source.
D) to keep membranes flexible at low temperatures.
E) as a building block of DNA and RNA molecules.
A) as a component of cell walls.
B) as a long-term energy source.
C) as a short-term energy source.
D) to keep membranes flexible at low temperatures.
E) as a building block of DNA and RNA molecules.
D
4
A weak acid may function as a
A) transfer group.
B) buffer.
C) hydroxyl donor.
D) cation.
E) salt.
A) transfer group.
B) buffer.
C) hydroxyl donor.
D) cation.
E) salt.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
5
The number of ________ of an element determines its atomic number.
A) protons
B) neutrons
C) electrons
D) valence electrons
E) isotopes
A) protons
B) neutrons
C) electrons
D) valence electrons
E) isotopes

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
6
The reverse of a dehydration synthesis reaction is a(n) ________ reaction.
A) anabolic
B) exchange
C) hydrolytic
D) endothermic
E) metabolic
A) anabolic
B) exchange
C) hydrolytic
D) endothermic
E) metabolic

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
7
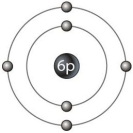 The atomic mass of this stable isotope atom (Figure 2.1) is
The atomic mass of this stable isotope atom (Figure 2.1) isA) 4.
B) 6.
C) 10.
D) 12.
E) cannot be determined from the available information

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is NOT a characteristic of phospholipids?
A) they are found in cellular membranes.
B) they can form micelles and bilayers.
C) they contain fatty acids that associate with water.
D) they contain a hydrophilic phosphate "head."
E) they contain two fatty acids and a phosphate functional group.
A) they are found in cellular membranes.
B) they can form micelles and bilayers.
C) they contain fatty acids that associate with water.
D) they contain a hydrophilic phosphate "head."
E) they contain two fatty acids and a phosphate functional group.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
9
An acid dissociates in water to release
A) hydrogen ion(s).
B) cation(s).
C) hydroxyl group(s).
D) anion(s).
E) both anions and hydrogen ions.
A) hydrogen ion(s).
B) cation(s).
C) hydroxyl group(s).
D) anion(s).
E) both anions and hydrogen ions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
10
The type(s) of bond(s) produced when atoms share electrons equally is/are
A) a nonpolar covalent bond.
B) a hydrogen bond.
C) an ionic bond.
D) a polar covalent bond.
E) both polar covalent and ionic bonds.
A) a nonpolar covalent bond.
B) a hydrogen bond.
C) an ionic bond.
D) a polar covalent bond.
E) both polar covalent and ionic bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is an atomic particle that has no electrical charge?
A) electron
B) neutron
C) element
D) proton
E) isotope
A) electron
B) neutron
C) element
D) proton
E) isotope

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
12
The type(s) of bond(s) produced when atoms with somewhat different electronegativities share electrons is/are
A) a nonpolar covalent bond.
B) a polar covalent bond.
C) an ionic bond.
D) a hydrogen bond.
E) both nonpolar covalent and ionic bonds.
A) a nonpolar covalent bond.
B) a polar covalent bond.
C) an ionic bond.
D) a hydrogen bond.
E) both nonpolar covalent and ionic bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
13
The carbon atoms in organic compounds typically form ________ with other atoms.
A) nonpolar covalent bonds
B) polar covalent bonds
C) ionic bonds
D) hydrogen bonds
E) either ionic or hydrogen bonds
A) nonpolar covalent bonds
B) polar covalent bonds
C) ionic bonds
D) hydrogen bonds
E) either ionic or hydrogen bonds

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
14
Nucleic acids, proteins, and complex carbohydrates are all produced by
A) hydrolytic reactions.
B) dehydration synthesis.
C) exchange reactions.
D) hydrogen bonding.
E) catabolic reactions.
A) hydrolytic reactions.
B) dehydration synthesis.
C) exchange reactions.
D) hydrogen bonding.
E) catabolic reactions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
15
Matter composed of a single type of atom is known as a(n)
A) element.
B) mineral.
C) molecule.
D) compound.
E) electron.
A) element.
B) mineral.
C) molecule.
D) compound.
E) electron.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
16
The chemical formula of the oxygen we require is O₂. It is
A) a compound.
B) an isotope.
C) an element.
D) a molecule.
E) both an element and a molecule.
A) a compound.
B) an isotope.
C) an element.
D) a molecule.
E) both an element and a molecule.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
17
A stable atom has ________ in its valence shell.
A) 4 electrons
B) 2 neutrons
C) 8 electrons
D) 8 protons
E) 10 electrons
A) 4 electrons
B) 2 neutrons
C) 8 electrons
D) 8 protons
E) 10 electrons

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is NOT a characteristic of saturated fats?
A) they are usually solid at room temperature.
B) they contain at least one double bond.
C) they are found in animals.
D) their fatty acids pack tightly together.
E) they are a form of stored energy.
A) they are usually solid at room temperature.
B) they contain at least one double bond.
C) they are found in animals.
D) their fatty acids pack tightly together.
E) they are a form of stored energy.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is an incorrect pairing?
A) electrolytes; anions
B) synthesis; endothermic
C) hydrolysis; hydrogen bonds
D) catabolism; exothermic
E) dehydration; anabolism
A) electrolytes; anions
B) synthesis; endothermic
C) hydrolysis; hydrogen bonds
D) catabolism; exothermic
E) dehydration; anabolism

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
20
Which parts of the atoms interact in a chemical reaction?
A) protons
B) neutrons
C) ions
D) electrons
E) isotopes
A) protons
B) neutrons
C) ions
D) electrons
E) isotopes

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
21
All amino acids contain what functional group(s)?
A) aldehyde
B) amino
C) ester
D) carboxyl
E) both amino and carboxyl
A) aldehyde
B) amino
C) ester
D) carboxyl
E) both amino and carboxyl

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
22
Cell walls containing ________ provide the best protection from drying.
A) polysaccharides
B) triglycerides
C) waxes
D) peptidoglycan
E) sterols
A) polysaccharides
B) triglycerides
C) waxes
D) peptidoglycan
E) sterols

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
23
All of the following are components of an amino acid EXCEPT a(n)
A) carboxyl group.
B) pentose group.
C) amino group.
D) α-carbon.
E) R group.
A) carboxyl group.
B) pentose group.
C) amino group.
D) α-carbon.
E) R group.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following statements about proteins is FALSE?
A) They are composed of amino acids.
B) They have multiple levels of structural organization.
C) They can be hydrophobic, hydrophilic, or both.
D) Their primary function is energy storage.
E) They are formed by dehydration synthesis reactions.
A) They are composed of amino acids.
B) They have multiple levels of structural organization.
C) They can be hydrophobic, hydrophilic, or both.
D) Their primary function is energy storage.
E) They are formed by dehydration synthesis reactions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following would NOT normally be found as a component of a cell's nucleic acids?
A) adenine deoxyribonucleotides
B) thymine deoxyribonucleotides
C) uracil deoxyribonucleotides
D) cytosine ribonucleotides
E) adenine ribonucleotides
A) adenine deoxyribonucleotides
B) thymine deoxyribonucleotides
C) uracil deoxyribonucleotides
D) cytosine ribonucleotides
E) adenine ribonucleotides

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
26
A(n) ________ is a compound that dissolves into anions and cations in water.
A) acid
B) buffer
C) base
D) salt
E) catalyst
A) acid
B) buffer
C) base
D) salt
E) catalyst

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
27
Synthesis reactions are commonly ________ reactions.
A) endothermic
B) exchange
C) exothermic
D) anabolic
E) hydrolytic
A) endothermic
B) exchange
C) exothermic
D) anabolic
E) hydrolytic

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is an accurate description of ATP?
A) ATP is a form of long term energy storage.
B) ATP is a compound formed of ionic bonds.
C) ATP does not readily react with other cellular macromolecules.
D) ATP serves as a recyclable energy for cells.
E) ATP is a structural component of DNA.
A) ATP is a form of long term energy storage.
B) ATP is a compound formed of ionic bonds.
C) ATP does not readily react with other cellular macromolecules.
D) ATP serves as a recyclable energy for cells.
E) ATP is a structural component of DNA.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is an incorrect pairing?
A) primary structure; amino acid sequence
B) secondary structure; disulfide bridges
C) tertiary structure; covalent bonds
D) quaternary structure; two or more polypeptides
E) secondary structure; β-pleated sheets
A) primary structure; amino acid sequence
B) secondary structure; disulfide bridges
C) tertiary structure; covalent bonds
D) quaternary structure; two or more polypeptides
E) secondary structure; β-pleated sheets

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
30
Lipids found in the cytoplasmic membranes of all eukaryotic cells are
A) polyunsaturated fats.
B) phospholipids.
C) steroids.
D) waxes.
E) triglycerides.
A) polyunsaturated fats.
B) phospholipids.
C) steroids.
D) waxes.
E) triglycerides.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
31
A(n) ________ is an arrangement of atoms found in a variety of macromolecules.
A) buffer
B) isotope
C) salt
D) stereoisomer
E) functional group
A) buffer
B) isotope
C) salt
D) stereoisomer
E) functional group

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is NOT characteristic of RNA?
A) It is a helical polymer.
B) It is usually double-stranded.
C) Its "backbone" is composed of pentoses and phosphates.
D) It contains both purines and pyrimidines.
E) It can function as a catalyst.
A) It is a helical polymer.
B) It is usually double-stranded.
C) Its "backbone" is composed of pentoses and phosphates.
D) It contains both purines and pyrimidines.
E) It can function as a catalyst.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is an example of a polysaccharide?
A) glycogen
B) glucose
C) fructose
D) deoxyribose
E) sucrose
A) glycogen
B) glucose
C) fructose
D) deoxyribose
E) sucrose

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
34
Proteins contain both acidic and basic R groups and can, therefore, function as
A) energy storage macromolecules.
B) structural macromolecules.
C) buffers.
D) catalysts.
E) genetic material.
A) energy storage macromolecules.
B) structural macromolecules.
C) buffers.
D) catalysts.
E) genetic material.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
35
A protein is a ________ of amino acids.
A) monomer
B) polymer
C) bilayer
D) solution
E) decomposition product
A) monomer
B) polymer
C) bilayer
D) solution
E) decomposition product

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
36
Hydrogen bonds are found in all of the following EXCEPT
A) between phosphates in ATP.
B) in α-helices.
C) between water molecules.
D) in the DNA double helix between nucleotides.
E) between the R groups of amino acids in proteins.
A) between phosphates in ATP.
B) in α-helices.
C) between water molecules.
D) in the DNA double helix between nucleotides.
E) between the R groups of amino acids in proteins.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
37
Tertiary and quaternary structure of proteins involves ________ bonds.
A) hydrogen
B) ionic
C) polar covalent
D) nonpolar covalent
E) ionic, hydrogen, polar, and nonpolar covalent
A) hydrogen
B) ionic
C) polar covalent
D) nonpolar covalent
E) ionic, hydrogen, polar, and nonpolar covalent

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is found in nucleic acids?
A) amines
B) carboxylic acid
C) purines
D) glycerol
E) R group
A) amines
B) carboxylic acid
C) purines
D) glycerol
E) R group

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
39
All of the following bases are found in RNA molecules EXCEPT
A) adenine.
B) thymine.
C) uracil.
D) cytosine.
E) guanine.
A) adenine.
B) thymine.
C) uracil.
D) cytosine.
E) guanine.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
40
The double-strands of DNA result from the formation of ________ between the bases.
A) covalent bonds
B) peptide bonds
C) ionic bonds
D) hydrogen bonds
E) α−1,4 bonds
A) covalent bonds
B) peptide bonds
C) ionic bonds
D) hydrogen bonds
E) α−1,4 bonds

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is an organic compound?
A) adenine
B) carbon dioxide
C) molecular oxygen
D) sodium chloride
E) water
A) adenine
B) carbon dioxide
C) molecular oxygen
D) sodium chloride
E) water

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
42
An atom or molecule becomes a(n) (anion/ion/cation) when it loses an electron to a more electronegative molecule.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
43
The side groups of amino acids can interact with each other and with other molecules.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
44
A molecule composed of carbon and hydrogen is a compound.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
45
Cell surface markers composed of both carbohydrate and lipid molecules are known as (glycoproteins/glycolipids/LPS).

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
46
The electron shells of atoms hold eight electrons each.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
47
Hydrogen bonds are stronger than covalent bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
48
A nucleotide with a single cyclic ring structure is a pyrimidine.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
49
A(n) (nonpolar/polar/ionic/hydrogen) bond is one in which electrons are shared equally between atoms.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
50
The smallest chemical units of matter are elements.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is found in RNA but not DNA?
A) adenine
B) cytosine
C) deoxyribose
D) guanine
E) uracil
A) adenine
B) cytosine
C) deoxyribose
D) guanine
E) uracil

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
52
Dehydration synthesis is a common feature of polymer production in cells.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
53
Denaturation of a protein is always permanent.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
54
The valence of an atom is determined by the total number of electrons it contains.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
55
DNA is composed of repeating units of sugars, phosphates, and nucleic acids. This is an example of a
A) polymer.
B) monomer.
C) salt.
D) micelle.
E) lipid.
A) polymer.
B) monomer.
C) salt.
D) micelle.
E) lipid.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
56
A reaction requires water as a reactant and produces heat. What type of reaction is likely to be involved?
A) an endothermic reaction
B) a dehydration reaction
C) an exchange reaction
D) a synthesis reaction
E) The answer cannot be determined from the available information.
A) an endothermic reaction
B) a dehydration reaction
C) an exchange reaction
D) a synthesis reaction
E) The answer cannot be determined from the available information.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
57
Salts are produced from exchange reactions in which acids and bases neutralize each other.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
58
Anna is conducting an experiment using a pH indicator that is red at low pH, green at neutral pH and purple at high pH. She starts with a green solution. When she adds compound X to her solution it turns purple. Then she adds compound Z to the solution and it turns green. She adds more Z, the solution remains green. These observations suggest X is ________ and Z is ________.
A) a base; a buffer
B) an acid; a base
C) a base; a strong acid
D) an acid; a buffer
E) a buffer; a base
A) a base; a buffer
B) an acid; a base
C) a base; a strong acid
D) an acid; a buffer
E) a buffer; a base

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
59
An unbranched polymer composed of simple sugars is a(n)
A) protein.
B) triglyceride.
C) starch.
D) glycoprotein.
E) amino acid.
A) protein.
B) triglyceride.
C) starch.
D) glycoprotein.
E) amino acid.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
60
Radioactive iodine is sometimes used to treat thyroid cancer. This is an example of the use of (isotopes/elements/ isomers) in medical treatment.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
61
The monomer of a nucleic acid is called a (nucleoside/nucleotide/base).

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
62
When a base dissolves in water it releases a(n) (electron/cation/hydrogen ion).

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
63
A saturated fatty acid contains (no/one/multiple) double bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
64
The folding of a polypeptide into a three-dimensional shape is its (secondary/tertiary/quaternary) structure.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
65
A nitrogenous base composed of two rings is a (purine/pyrimidine/ribose).

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
66
Jim adds an acid to a solution, but finds the pH has not changed afterward. This suggests the solution contains a(n) (anion/buffer/salt).

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
67
A chemical reaction in which a water molecule is a reactant is known as a (dehydration/endothermic/hydrolysis) reaction.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
68
The isotopes of an element vary in the number of (electrons/neutrons/protons) in the atom.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
69
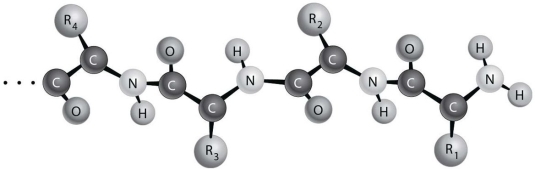
Figure 2.2 depicts the (primary/secondary/tertiary) structure of a protein.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
70
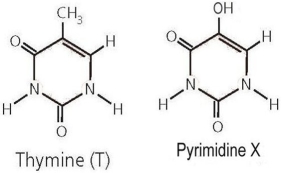
Consider the structure of thymine, shown on the left in Figure 2.3 above, and compare to the structure of pyrimidine X on the right. What would be the impact if X is incorporated into the structure of a DNA strand in place of thymine?

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
71
A(n) (catalyst/enzyme) is any molecule that speeds up a chemical reaction.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
72
Discuss the importance of hydrogen bonds in the chemistry of the cell.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
73
Max is exploring the properties of various compounds. Some of his explorations involve the use of a pH indicator that is red at low pH, yellow-green at neutral pH and blue to purple at high pH. He sets up several tubes containing water and the pH indicator and then begins to add some of the compounds (L, M, and N) he is characterizing in various combinations. His results are shown on the following table.
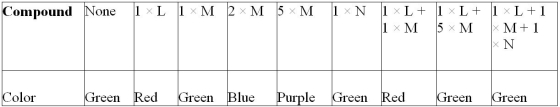
What can Max conclude about his compounds based on these results? Describe the likely events in terms of hydrogen and hydroxyl ions.

What can Max conclude about his compounds based on these results? Describe the likely events in terms of hydrogen and hydroxyl ions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
74
Describe the chemical properties of phospholipids that account for their behavior in water.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
75
The DNA double helix is held together by (covalent/ionic/hydrogen) bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
76
Compare and contrast synthesis reactions with decomposition reactions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck



