Deck 7: Alkyl Halides and Elimination Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/9
Play
Full screen (f)
Deck 7: Alkyl Halides and Elimination Reactions
1
Classify each alkene in vitamin D3 labeled I, II, III by the number of carbon substituents bonded to the double bond. 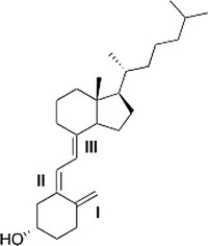
A) I = Monosubstituted; II = disubstituted; III = trisubstituted
B) I = Disubstituted; II = disubstituted; III = trisubstituted
C) I = Disubstituted; II = trisubstituted; III = disubstituted
D) I = Disubstituted; II = trisubstituted; III = trisubstituted

A) I = Monosubstituted; II = disubstituted; III = trisubstituted
B) I = Disubstituted; II = disubstituted; III = trisubstituted
C) I = Disubstituted; II = trisubstituted; III = disubstituted
D) I = Disubstituted; II = trisubstituted; III = trisubstituted
I = Disubstituted; II = trisubstituted; III = trisubstituted
2
Which of the following statements about an E1 mechanism is true?
A) The reaction is slowest with tertiary substrates.
B) The reaction follows second-order kinetics.
C) The identity of the leaving group affects the rate of reaction.
D) Polar aprotic solvents favor the E1 mechanism.
A) The reaction is slowest with tertiary substrates.
B) The reaction follows second-order kinetics.
C) The identity of the leaving group affects the rate of reaction.
D) Polar aprotic solvents favor the E1 mechanism.
The identity of the leaving group affects the rate of reaction.
3
What is the product of the following reaction? 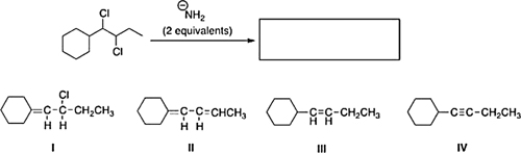
A) III
B) II
C) IV
D) I

A) III
B) II
C) IV
D) I
IV
4
What is the major product of the following reaction? 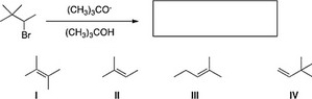
A) III
B) II
C) I
D) IV

A) III
B) II
C) I
D) IV

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following E2 reaction. What rate equation would be observed for this reaction? ![<strong>Consider the following E2 reaction. What rate equation would be observed for this reaction? </strong> A) Rate =k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>)<sub>3</sub>] B) Rate =k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br]<sup>2</sup>[KOC(CH<sub>3</sub>)<sub>3</sub>] C) Rate =k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br] D) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>)<sub>3</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf4_eadc_862d_49cab86c3690_TBMG1035_00.jpg)
A) Rate =k[CH3CH2CH2Br][KOC(CH3)3]
B) Rate =k[CH3CH2CH2Br]2[KOC(CH3)3]
C) Rate =k[CH3CH2CH2Br]
D) Rate = k[CH3CH2CH2Br][KOC(CH3)3]2
![<strong>Consider the following E2 reaction. What rate equation would be observed for this reaction? </strong> A) Rate =k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>)<sub>3</sub>] B) Rate =k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br]<sup>2</sup>[KOC(CH<sub>3</sub>)<sub>3</sub>] C) Rate =k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br] D) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>)<sub>3</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf4_eadc_862d_49cab86c3690_TBMG1035_00.jpg)
A) Rate =k[CH3CH2CH2Br][KOC(CH3)3]
B) Rate =k[CH3CH2CH2Br]2[KOC(CH3)3]
C) Rate =k[CH3CH2CH2Br]
D) Rate = k[CH3CH2CH2Br][KOC(CH3)3]2

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
6
What is the product of the following reaction? 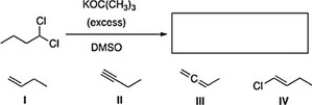
A) I
B) IV
C) III
D) II

A) I
B) IV
C) III
D) II

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements about an E1 mechanism isnot true?
A) The reaction follows first-order kinetics.
B) Stronger bases favor the E1 reaction.
C) The reaction is fastest with tertiary alkyl halides.
D) A better leaving group makes the reaction rate increase.
A) The reaction follows first-order kinetics.
B) Stronger bases favor the E1 reaction.
C) The reaction is fastest with tertiary alkyl halides.
D) A better leaving group makes the reaction rate increase.

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about the mechanism of an E2 reaction isnot true?
A) It is fastest with tertiary halides.
B) All bonds are broken and formed in a single step.
C) It exhibits first-order kinetics.
D) A better leaving group should make a faster reaction.
A) It is fastest with tertiary halides.
B) All bonds are broken and formed in a single step.
C) It exhibits first-order kinetics.
D) A better leaving group should make a faster reaction.

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
9
What is the product of the following reaction? 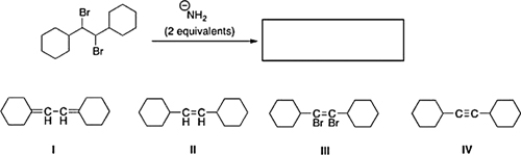
A) II
B) III
C) IV
D) I

A) II
B) III
C) IV
D) I

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck



