Deck 6: Alkyl Halides and Nucleophilic Substitution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/11
Play
Full screen (f)
Deck 6: Alkyl Halides and Nucleophilic Substitution
1
Given the following substitution reaction, what would be the effect of changing the solvent from CH3OH to (CH3)2S=O?
CH3(CH2)5Br + NaOH as it goes to CH3(CH2)5OH + Br?
A) The rate would decrease because SN2 reactions are favored by polar aprotic solvents.
B) The rate would increase because SN1 reactions are favored by polar protic solvents.
C) The rate would increase because SN2 reactions are favored by polar aprotic solvents.
D) The rate would decrease because SN1 reactions are favored by polar protic solvents.
CH3(CH2)5Br + NaOH as it goes to CH3(CH2)5OH + Br?
A) The rate would decrease because SN2 reactions are favored by polar aprotic solvents.
B) The rate would increase because SN1 reactions are favored by polar protic solvents.
C) The rate would increase because SN2 reactions are favored by polar aprotic solvents.
D) The rate would decrease because SN1 reactions are favored by polar protic solvents.
The rate would increase because SN2 reactions are favored by polar aprotic solvents.
2
Which of the following statements about the Hammond postulate isnot true?
A) In endothermic reactions, the transition state is closer in energy to the products.
B) The Hammond postulate provides a qualitative estimate of the energy of a transition state.
C) The Hammond postulate provides a quantitative estimate of the energy of a transition state.
D) In exothermic reactions, the transition state is closer in energy to the reactants.
A) In endothermic reactions, the transition state is closer in energy to the products.
B) The Hammond postulate provides a qualitative estimate of the energy of a transition state.
C) The Hammond postulate provides a quantitative estimate of the energy of a transition state.
D) In exothermic reactions, the transition state is closer in energy to the reactants.
The Hammond postulate provides a quantitative estimate of the energy of a transition state.
3
Which is the best reagent to carry out the following organic synthesis? 
A) NaOH, DMSO
B) NaOMe, DMSO
C) H2O
D) MeOH

A) NaOH, DMSO
B) NaOMe, DMSO
C) H2O
D) MeOH
NaOMe, DMSO
4
Which of the following statements about the Hammond postulate is true?
A) In an endothermic reaction, the more stable product forms faster.
B) In an endothermic reaction, the less stable product forms faster.
C) In an endothermic reaction, the activation energy, Ea, is similar for both products.
D) In an exothermic reaction, lowering the energy of the transition state increases the activation energy, Ea.
A) In an endothermic reaction, the more stable product forms faster.
B) In an endothermic reaction, the less stable product forms faster.
C) In an endothermic reaction, the activation energy, Ea, is similar for both products.
D) In an exothermic reaction, lowering the energy of the transition state increases the activation energy, Ea.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements isnot true?
A) Equilibrium favors the products of the nucleophilic substitution when the leaving group is a stronger base than the nucleophile.
B) All good leaving groups are weak bases with strong conjugate acids.
C) Left-to-right across a row of the periodic table, leaving group ability increases.
D) Down a column of the periodic table, leaving group ability increases.
A) Equilibrium favors the products of the nucleophilic substitution when the leaving group is a stronger base than the nucleophile.
B) All good leaving groups are weak bases with strong conjugate acids.
C) Left-to-right across a row of the periodic table, leaving group ability increases.
D) Down a column of the periodic table, leaving group ability increases.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
6
What is the IUPAC name of the following compound? 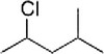
A) 2-Chloro-4-methylpentane
B) 2-Chloro-1-isopropylpropane
C) 2-Chloro-2-methylpentane
D) 2-Methyl-4-chloropentane

A) 2-Chloro-4-methylpentane
B) 2-Chloro-1-isopropylpropane
C) 2-Chloro-2-methylpentane
D) 2-Methyl-4-chloropentane

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
7
Which is the best reagent to carry out the following organic synthesis? 
A) H2O
B) H2S
C) HCl
D) MeOH

A) H2O
B) H2S
C) HCl
D) MeOH

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements explain why aryl halides and vinyl halidesdo not undergo nucleophilic substitution by either the SN1 or SN2 mechanism?
A) They don't undergo SN1 reactions because a higher percents-character makes the bond longer and stronger.
B) They don't undergo SN1 reactions because the carbocation is highly electronegative.
C) They don't undergo SN2 reactions because a higher percents-character makes the bond shorter and stronger.
D) They don't undergo SN2 reactions because heterolysis of the C-X bond forms a highly unstable carbocation.
A) They don't undergo SN1 reactions because a higher percents-character makes the bond longer and stronger.
B) They don't undergo SN1 reactions because the carbocation is highly electronegative.
C) They don't undergo SN2 reactions because a higher percents-character makes the bond shorter and stronger.
D) They don't undergo SN2 reactions because heterolysis of the C-X bond forms a highly unstable carbocation.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements about SN2 reactions is true?
A) The mechanism is a two-step process.
B) The rate of reaction is dependent on just the substrate.
C) Displacement occurs with inversion of configuration.
D) The fastest reaction will occur with a tertiary alkyl halide.
A) The mechanism is a two-step process.
B) The rate of reaction is dependent on just the substrate.
C) Displacement occurs with inversion of configuration.
D) The fastest reaction will occur with a tertiary alkyl halide.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is true?
A) Down a column of the periodic table, leaving group ability decreases.
B) The conjugate bases of strong acids are good leaving groups.
C) All good leaving groups are strong bases with weak conjugate acids.
D) Left-to-right across a row of the periodic table, leaving group ability decreases.
A) Down a column of the periodic table, leaving group ability decreases.
B) The conjugate bases of strong acids are good leaving groups.
C) All good leaving groups are strong bases with weak conjugate acids.
D) Left-to-right across a row of the periodic table, leaving group ability decreases.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
11
The reaction oftert-butyl bromide, (CH3)3CBr, with ethanol affords the substitution producttert-butyl ethyl ether, (CH3)3COCH2CH3, in acidic conditions. What would happen to the rate of the reaction if the concentration of ethanol was doubled?
A) The rate increases by a factor of 4.
B) The rate increases by a factor of 2.
C) The rate remains the same.
D) The rate decreases by a factor of 2.
A) The rate increases by a factor of 4.
B) The rate increases by a factor of 2.
C) The rate remains the same.
D) The rate decreases by a factor of 2.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck



