Deck 5: Understanding Organic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/16
Play
Full screen (f)
Deck 5: Understanding Organic Reactions
1
Which of the following statements isnot true?
A) DH° determines the height of the energy barrier.
B) The larger the activation energy, the slower the reaction.
C) The lower the activation energy, the faster the reaction.
D) Two reactions can have identical values for DH° but very different Ea values.
A) DH° determines the height of the energy barrier.
B) The larger the activation energy, the slower the reaction.
C) The lower the activation energy, the faster the reaction.
D) Two reactions can have identical values for DH° but very different Ea values.
DH° determines the height of the energy barrier.
2
Which reaction is slowest? 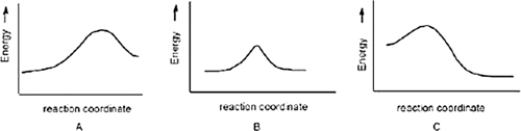
A) B
B) C
C) A

A) B
B) C
C) A
A
3
Which of the following statements about the equilibrium constant,Keq, is true?
A) The size ofKeq tells about the position of equilibrium.
B) WhenKeq > 1, the equilibrium favors the reactants.
C) For a reaction to be useful, the equilibrium must favor the reactants.
D) WhenKeq < 1, the equilibrium favors the products.
A) The size ofKeq tells about the position of equilibrium.
B) WhenKeq > 1, the equilibrium favors the reactants.
C) For a reaction to be useful, the equilibrium must favor the reactants.
D) WhenKeq < 1, the equilibrium favors the products.
The size ofKeq tells about the position of equilibrium.
4
Which of the following statements isnot true?
A) The bond dissociation energy for bond formation is always negative.
B) The bond dissociation energy for bond breaking is always negative.
C) Bond making is exothermic.
D) Bond breaking is endothermic.
A) The bond dissociation energy for bond formation is always negative.
B) The bond dissociation energy for bond breaking is always negative.
C) Bond making is exothermic.
D) Bond breaking is endothermic.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
5
The equilibrium constant for the conversion of A to D is predicted to be which of the following? 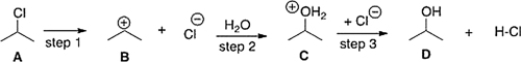
A) Keq < 1
B) Cannot be determined from the information provided
C) Keq > 1
D) Keq = 1

A) Keq < 1
B) Cannot be determined from the information provided
C) Keq > 1
D) Keq = 1

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements about enzymes is true?
A) Enzymes shift the equilibrium to favor the product.
B) Enzymes lower the transition state for the rate-determining step.
C) Enzymes decrease the equilibrium constant.
D) Enzymes increase the activation energy for a reaction.
A) Enzymes shift the equilibrium to favor the product.
B) Enzymes lower the transition state for the rate-determining step.
C) Enzymes decrease the equilibrium constant.
D) Enzymes increase the activation energy for a reaction.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate Ea for the conversion of C to B.
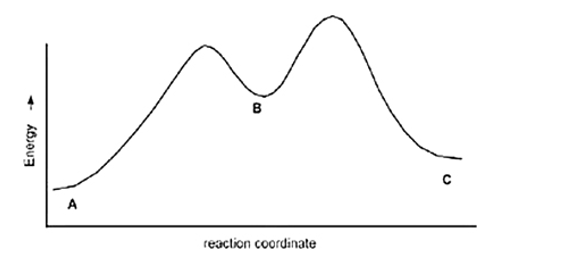 Ea (A to B)=+10 Kcal
Ea (A to B)=+10 Kcal
Ea (B to C)=+4 Kcal
DH (A to B)=+8 Kcal
DH (B to C)=-5 Kcal
A) +7 kcal
B) None of these
C) +3 kcal
D)+9 Kcal
 Ea (A to B)=+10 Kcal
Ea (A to B)=+10 KcalEa (B to C)=+4 Kcal
DH (A to B)=+8 Kcal
DH (B to C)=-5 Kcal
A) +7 kcal
B) None of these
C) +3 kcal
D)+9 Kcal

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about substitution reactions is true?
A) One ? bond breaks and another forms at a different carbon atom.
B) One ? bond breaks and another forms at the same carbon atom.
C) Substitution reactions involve ? bonds.
D) Substitution reactions involve ? bonds.
A) One ? bond breaks and another forms at a different carbon atom.
B) One ? bond breaks and another forms at the same carbon atom.
C) Substitution reactions involve ? bonds.
D) Substitution reactions involve ? bonds.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
9
How many steps are there in a concerted mechanism?
A) 1
B) 2
C) 4
D) 3
A) 1
B) 2
C) 4
D) 3

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is true?
A) Bond dissociation energies decrease down a column of the periodic table.
B) Bond dissociation energies increase down a column of the periodic table.
C) When DH° is positive, more energy is released in forming bonds than is needed to break bonds.
D) When DH° is negative, more energy is needed to break bonds than is released in forming bonds.
A) Bond dissociation energies decrease down a column of the periodic table.
B) Bond dissociation energies increase down a column of the periodic table.
C) When DH° is positive, more energy is released in forming bonds than is needed to break bonds.
D) When DH° is negative, more energy is needed to break bonds than is released in forming bonds.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
11
The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism:
What is the rate equation for this reaction if the first step is rate determining?
![<strong>The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: What is the rate equation for this reaction if the first step is rate determining? </strong> A) Rate = k [acetyl chloride] [<sup>?</sup>OCH<sub>3</sub>] B) Rate = k [acetyl chloride] C) Rate = k [<sup>?</sup>OCH<sub>3</sub>] D) Rate = k [acetyl chloride] [<sup>?</sup>OCH<sub>3</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf5_ae35_862d_b3ca016c109f_TBMG1035_00.jpg)
A) Rate = k [acetyl chloride] [?OCH3]
B) Rate = k [acetyl chloride]
C) Rate = k [?OCH3]
D) Rate = k [acetyl chloride] [?OCH3]2
What is the rate equation for this reaction if the first step is rate determining?
![<strong>The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: What is the rate equation for this reaction if the first step is rate determining? </strong> A) Rate = k [acetyl chloride] [<sup>?</sup>OCH<sub>3</sub>] B) Rate = k [acetyl chloride] C) Rate = k [<sup>?</sup>OCH<sub>3</sub>] D) Rate = k [acetyl chloride] [<sup>?</sup>OCH<sub>3</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf5_ae35_862d_b3ca016c109f_TBMG1035_00.jpg)
A) Rate = k [acetyl chloride] [?OCH3]
B) Rate = k [acetyl chloride]
C) Rate = k [?OCH3]
D) Rate = k [acetyl chloride] [?OCH3]2

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
12
The following is an energy diagram for the conversion of A ? B ?C. The energies of activation and DH's for each step are also given. Calculate DH overall as shown on the energy diagram for A ? B ? C.
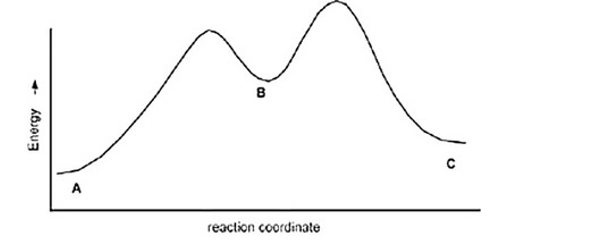
Ea (A ?B)=+10 Kcal
Ea (B?C)=+4 Kcal
DH (A ?B)=+8 Kcal
DH (B ?C)=-5 Kcal
A) +9 kcal
B) +7 kcal
C)None of these
D) +3 Kcal

Ea (A ?B)=+10 Kcal
Ea (B?C)=+4 Kcal
DH (A ?B)=+8 Kcal
DH (B ?C)=-5 Kcal
A) +9 kcal
B) +7 kcal
C)None of these
D) +3 Kcal

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements about equilibrium is true?
A) Equilibrium favors the products when they are more stable than the starting material of a reaction.
B) Equilibrium favors the reactants when the energy of the product is lower than the energy of the reactants.
C) Equilibrium favors the products when the energy of the products is higher than the energy of the reactants.
D) Equilibrium favors the products when they are less stable than the starting material of a reaction.
A) Equilibrium favors the products when they are more stable than the starting material of a reaction.
B) Equilibrium favors the reactants when the energy of the product is lower than the energy of the reactants.
C) Equilibrium favors the products when the energy of the products is higher than the energy of the reactants.
D) Equilibrium favors the products when they are less stable than the starting material of a reaction.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
14
A reaction that results in the formation of a carbocation is most likely to occur from the following:
A) None of these are correct.
B) Heterolytic bond cleavage
C) All of the above
D) Homolytic bond cleavage
A) None of these are correct.
B) Heterolytic bond cleavage
C) All of the above
D) Homolytic bond cleavage

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
15
If the conversion of A to B is slow and B to C is fast, what is the rate equation for this reaction? ![<strong>If the conversion of A to B is slow and B to C is fast, what is the rate equation for this reaction? </strong> A) Rate =k[(CH<sub>3</sub>)<sub>2</sub>CH]<sup>+</sup>[H<sub>2</sub>O] B) Rate =k[(CH<sub>3</sub>)<sub>2</sub>CHCl] C) Rate =k[(CH<sub>3</sub>)<sub>2</sub>CHCl][H<sub>2</sub>O] D) Rate =k[(CH<sub>3</sub>)<sub>2</sub>CH]<sup>+</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf5_d547_862d_493b25a95154_TBMG1035_00.jpg)
A) Rate =k[(CH3)2CH]+[H2O]
B) Rate =k[(CH3)2CHCl]
C) Rate =k[(CH3)2CHCl][H2O]
D) Rate =k[(CH3)2CH]+
![<strong>If the conversion of A to B is slow and B to C is fast, what is the rate equation for this reaction? </strong> A) Rate =k[(CH<sub>3</sub>)<sub>2</sub>CH]<sup>+</sup>[H<sub>2</sub>O] B) Rate =k[(CH<sub>3</sub>)<sub>2</sub>CHCl] C) Rate =k[(CH<sub>3</sub>)<sub>2</sub>CHCl][H<sub>2</sub>O] D) Rate =k[(CH<sub>3</sub>)<sub>2</sub>CH]<sup>+</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cf5_d547_862d_493b25a95154_TBMG1035_00.jpg)
A) Rate =k[(CH3)2CH]+[H2O]
B) Rate =k[(CH3)2CHCl]
C) Rate =k[(CH3)2CHCl][H2O]
D) Rate =k[(CH3)2CH]+

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements about bond breaking isnot true?
A) Heterolysis involves unequal sharing of bonding electrons by atoms.
B) Homolysis generates uncharged reactive intermediates with unpaired electrons.
C) Homolysis require energy but heterolysis does not require energy.
D) Heterolysis generates charged intermediates.
A) Heterolysis involves unequal sharing of bonding electrons by atoms.
B) Homolysis generates uncharged reactive intermediates with unpaired electrons.
C) Homolysis require energy but heterolysis does not require energy.
D) Heterolysis generates charged intermediates.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck



