Deck 23: Carbohydrates
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/8
Play
Full screen (f)
Deck 23: Carbohydrates
1
Amylose is a polysaccharide with ________ linkages.
A) a-1,4'-glycosidic
B) a-1,6'-glycosidic
C) b-1,4'-glycosidic
D) b-1,6'-glycosidic
A) a-1,4'-glycosidic
B) a-1,6'-glycosidic
C) b-1,4'-glycosidic
D) b-1,6'-glycosidic
a-1,4'-glycosidic
2
Cellulose is a polysaccharide with ________ linkages.
A) a-1,4'-glycosidic
B) b-1,6'-glycosidic
C) b-1,4'-glycosidic
D) a-1,6'-glycosidic
A) a-1,4'-glycosidic
B) b-1,6'-glycosidic
C) b-1,4'-glycosidic
D) a-1,6'-glycosidic
b-1,4'-glycosidic
3
Which of the following is D-glucose? 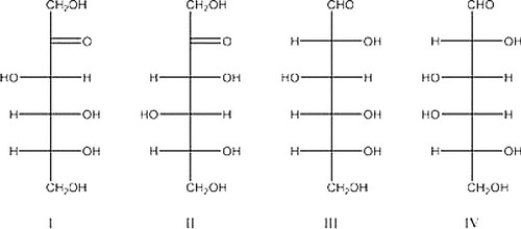
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
III
4
Which of the following is D-fructose? 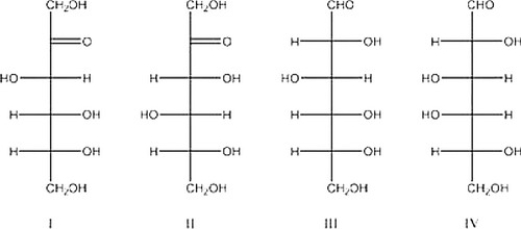
A) I
B) IV
C) II
D) III

A) I
B) IV
C) II
D) III

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
5
What are the products of the following reaction? 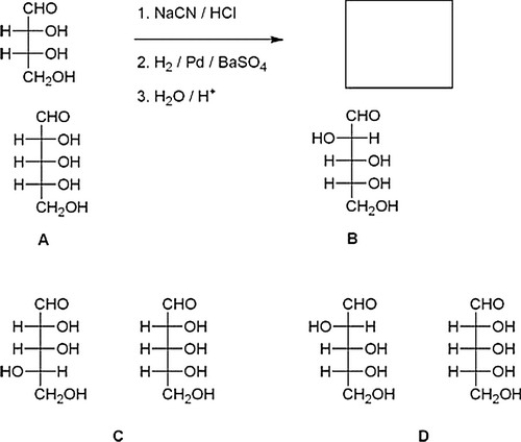
A) D
B) A
C) C
D) B

A) D
B) A
C) C
D) B

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
6
After a series of Kiliani-Fischer syntheses on D-glyceraldehyde, an unknown sugar was isolated from the reaction mixture. The following experimental data was obtained. What is the structure of this unknown sugar?
Data:
- Molecular formula: C6H12O6
- Reacts with phenylhydrazine to give an osazone, mp 178°C.
- Reacts with HNO3 to give an optically active aldaric acid.
- Wohl degradation followed by HNO3 oxidation gives an optically inactive aldaric acid.
- Two Wohl degradations followed by HNO3 oxidation give a meso-tartaric acid.
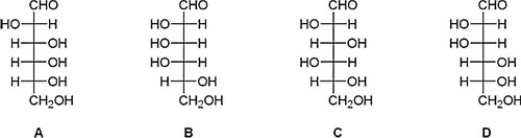
A) D
B) C
C) A
D) B
Data:
- Molecular formula: C6H12O6
- Reacts with phenylhydrazine to give an osazone, mp 178°C.
- Reacts with HNO3 to give an optically active aldaric acid.
- Wohl degradation followed by HNO3 oxidation gives an optically inactive aldaric acid.
- Two Wohl degradations followed by HNO3 oxidation give a meso-tartaric acid.

A) D
B) C
C) A
D) B

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
7
What type of linkage is present in chitin?
A) beta-1,2'-glycosidic linkage
B) alpha-1,2'-glycosidic linkage
C) alpha-1,4'-glycosidic linkage
D) beta-1,4'-glycosidic linkage
A) beta-1,2'-glycosidic linkage
B) alpha-1,2'-glycosidic linkage
C) alpha-1,4'-glycosidic linkage
D) beta-1,4'-glycosidic linkage

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
8
What is the composition of sucrose?
A) Repeating unit of glucose units joined in a b-1,4'-glycosidic linkage
B) Two glucose units with a a-1,4'-glycoside bond
C) One galactose and one glucose unit with a b-1,4'-glycoside bond
D) One glucose and one fructose unit with a a-glycosidic bond to the 2' carbon of a fructofuranose ring
A) Repeating unit of glucose units joined in a b-1,4'-glycosidic linkage
B) Two glucose units with a a-1,4'-glycoside bond
C) One galactose and one glucose unit with a b-1,4'-glycoside bond
D) One glucose and one fructose unit with a a-glycosidic bond to the 2' carbon of a fructofuranose ring

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck



