Deck 21: Amines
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/16
Play
Full screen (f)
Deck 21: Amines
1
Which of the following are radical inhibitors?
A) None of these are correct.
B) Vitamin E
C) BHT
D) Both of these choices are correct.
A) None of these are correct.
B) Vitamin E
C) BHT
D) Both of these choices are correct.
Both of these choices are correct.
2
The proposed reaction between chlorofluorocarbons and ozone involves what type of mechanism?
A) Substitution mechanism
B) Elimination mechanism
C) Electrophilic aromatic substitution
D) Radical mechanism
A) Substitution mechanism
B) Elimination mechanism
C) Electrophilic aromatic substitution
D) Radical mechanism
Radical mechanism
3
The proposed reaction of the oxidation of unsaturated lipids involves what type of mechanism?
A) Substitution mechanism
B) Radical mechanism
C) Elimination mechanism
D) Electrophilic aromatic substitution
A) Substitution mechanism
B) Radical mechanism
C) Elimination mechanism
D) Electrophilic aromatic substitution
Radical mechanism
4
Which of the following statements about the stereochemistry of halogenation reactions is true?
A) The configuration at a stereogenic center of a product must change even if a reaction does not occur at a stereogenic center.
B) An achiral starting material always gives an achiral product only.
C) An achiral starting material always gives either an achiral or a racemic product.
D) An achiral starting material always gives a racemic product only.
A) The configuration at a stereogenic center of a product must change even if a reaction does not occur at a stereogenic center.
B) An achiral starting material always gives an achiral product only.
C) An achiral starting material always gives either an achiral or a racemic product.
D) An achiral starting material always gives a racemic product only.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
5
What is the product of the following reaction? 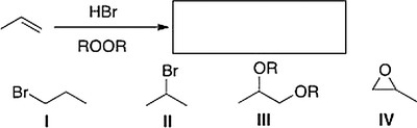
A) III
B) IV
C) II
D) I

A) III
B) IV
C) II
D) I

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
6
What is the product of the following reaction? 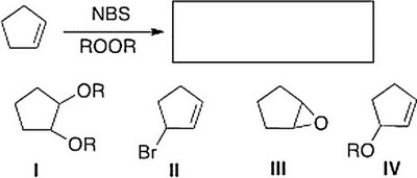
A) II
B) I
C) IV
D) III

A) II
B) I
C) IV
D) III

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements about the propagation steps in the chlorination of ethane is true?
A) The second of the propagation steps is rate-determining because its transition state is at higher energy.
B) The first of the propagation steps is rate-determining because its transition state is at lower energy.
C) Radical chlorination consists of two propagation steps.
D) The energy diagram for the propagation steps has three energy barriers.
A) The second of the propagation steps is rate-determining because its transition state is at higher energy.
B) The first of the propagation steps is rate-determining because its transition state is at lower energy.
C) Radical chlorination consists of two propagation steps.
D) The energy diagram for the propagation steps has three energy barriers.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about carbon radicals isnot true?
A) A carbon radical issp2 hybridized.
B) Carbon radicals are classified as primary, secondary, tertiary, or quaternary.
C) The geometry of a carbon radical is trigonal planar.
D) The unhybridizedp orbital in a carbon radical contains the unpaired electron.
A) A carbon radical issp2 hybridized.
B) Carbon radicals are classified as primary, secondary, tertiary, or quaternary.
C) The geometry of a carbon radical is trigonal planar.
D) The unhybridizedp orbital in a carbon radical contains the unpaired electron.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements about radicals and radical reactions isnot true?
A) A radical is formed by homolysis of a covalent bond.
B) Most radicals are unstable.
C) A radical contains an atom that has an octet of electrons.
D) Half-headed arrows are used to show the movement of lone electrons.
A) A radical is formed by homolysis of a covalent bond.
B) Most radicals are unstable.
C) A radical contains an atom that has an octet of electrons.
D) Half-headed arrows are used to show the movement of lone electrons.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements about chlorination is true?
A) The transition state resembles the product.
B) The more stable radical is formed faster.
C) A mixture of products results.
D) The rate-determining step in chlorination is endothermic.
A) The transition state resembles the product.
B) The more stable radical is formed faster.
C) A mixture of products results.
D) The rate-determining step in chlorination is endothermic.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
11
A possible reaction of ethane with chlorine is shown below.
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1]. Bond dissociation energies (kcal/mol): \begin{array}{|l|l|} \hline \text { Bond A-B } & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3}-\mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3}-\mathrm{Cl} & 84 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A)-5 kcal/mol B) None of the choices are correct. C) +58 kcal/mol D) -28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfb_f023_862d_b3fc3c178521_TBMG1035_00.jpg)
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1].
Bond dissociation energies (kcal/mol):
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1]. Bond dissociation energies (kcal/mol): \begin{array}{|l|l|} \hline \text { Bond A-B } & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3}-\mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3}-\mathrm{Cl} & 84 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A)-5 kcal/mol B) None of the choices are correct. C) +58 kcal/mol D) -28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfb_f024_862d_adf53f905e54_TBMG1035_00.jpg)
A)-5 kcal/mol
B) None of the choices are correct.
C) +58 kcal/mol
D) -28 kcal/mol
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1]. Bond dissociation energies (kcal/mol): \begin{array}{|l|l|} \hline \text { Bond A-B } & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3}-\mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3}-\mathrm{Cl} & 84 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A)-5 kcal/mol B) None of the choices are correct. C) +58 kcal/mol D) -28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfb_f023_862d_b3fc3c178521_TBMG1035_00.jpg)
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1].
Bond dissociation energies (kcal/mol):
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1]. Bond dissociation energies (kcal/mol): \begin{array}{|l|l|} \hline \text { Bond A-B } & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3}-\mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3}-\mathrm{Cl} & 84 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A)-5 kcal/mol B) None of the choices are correct. C) +58 kcal/mol D) -28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfb_f024_862d_adf53f905e54_TBMG1035_00.jpg)
A)-5 kcal/mol
B) None of the choices are correct.
C) +58 kcal/mol
D) -28 kcal/mol

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
12
A possible reaction of ethane with chlorine is shown below.
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2]. Bond dissociation energies (kcal/mol): \begin{array}{|ll|} \hline \text { Bond } \mathrm{A}-\mathrm{B} & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3} \mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{Cl} & 81 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) +58 kcal/mol B) -28 kcal/mol C)-5 kcal/mol D) None of the choices are correct.](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_1736_862d_a16448f336a1_TBMG1035_00.jpg)
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2].
Bond dissociation energies (kcal/mol):
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2]. Bond dissociation energies (kcal/mol): \begin{array}{|ll|} \hline \text { Bond } \mathrm{A}-\mathrm{B} & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3} \mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{Cl} & 81 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) +58 kcal/mol B) -28 kcal/mol C)-5 kcal/mol D) None of the choices are correct.](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_1737_862d_13a7c1768b84_TBMG1035_00.jpg)
A) +58 kcal/mol
B) -28 kcal/mol
C)-5 kcal/mol
D) None of the choices are correct.
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2]. Bond dissociation energies (kcal/mol): \begin{array}{|ll|} \hline \text { Bond } \mathrm{A}-\mathrm{B} & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3} \mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{Cl} & 81 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) +58 kcal/mol B) -28 kcal/mol C)-5 kcal/mol D) None of the choices are correct.](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_1736_862d_a16448f336a1_TBMG1035_00.jpg)
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2].
Bond dissociation energies (kcal/mol):
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2]. Bond dissociation energies (kcal/mol): \begin{array}{|ll|} \hline \text { Bond } \mathrm{A}-\mathrm{B} & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3} \mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{Cl} & 81 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) +58 kcal/mol B) -28 kcal/mol C)-5 kcal/mol D) None of the choices are correct.](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_1737_862d_13a7c1768b84_TBMG1035_00.jpg)
A) +58 kcal/mol
B) -28 kcal/mol
C)-5 kcal/mol
D) None of the choices are correct.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
13
What is the product of the following reaction? 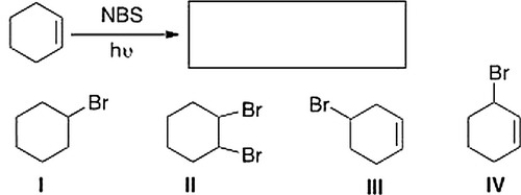
A) II
B) III
C) I
D) IV

A) II
B) III
C) I
D) IV

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
14
A possible reaction of ethane with chlorine is shown below.
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________.
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________. </strong> A) Only [1] and [2] B) Only [2] and [3] C) Only [3] D) Only [1] and [3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_173a_862d_83490f3df3f7_TBMG1035_00.jpg)
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________. </strong> A) Only [1] and [2] B) Only [2] and [3] C) Only [3] D) Only [1] and [3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_173b_862d_e53c42782ac5_TBMG1035_00.jpg)
A) Only [1] and [2]
B) Only [2] and [3]
C) Only [3]
D) Only [1] and [3]
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________.
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________. </strong> A) Only [1] and [2] B) Only [2] and [3] C) Only [3] D) Only [1] and [3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_173a_862d_83490f3df3f7_TBMG1035_00.jpg)
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________. </strong> A) Only [1] and [2] B) Only [2] and [3] C) Only [3] D) Only [1] and [3]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_173b_862d_e53c42782ac5_TBMG1035_00.jpg)
A) Only [1] and [2]
B) Only [2] and [3]
C) Only [3]
D) Only [1] and [3]

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements about radicals is true?
A) The higher the bond dissociation energy for a C-H bond, the more stable the resulting carbon radical.
B) Cleavage of a stronger bond forms the more stable radical.
C) The stability of a radical increases as the number of alkyl groups bonded to the radical carbon decreases.
D) Less stable radicals generally do not rearrange to more stable radicals.
A) The higher the bond dissociation energy for a C-H bond, the more stable the resulting carbon radical.
B) Cleavage of a stronger bond forms the more stable radical.
C) The stability of a radical increases as the number of alkyl groups bonded to the radical carbon decreases.
D) Less stable radicals generally do not rearrange to more stable radicals.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements is (are) true about free radical halogenation of alkanes?
A) The chain-initiating step involves cleavage of a carbon-hydrogen bond to afford a carbon radical and a hydrogen atom.
B) The first of the chain-propagating steps is rate-determining.
C) The reaction proceeds by way of a flatsp2 hybridized free radical.
D) Statements (The first of the chain-propagating steps is rate-determining) and (The reaction proceeds by way of a flatsp2 hybridized free radical) are both true.
A) The chain-initiating step involves cleavage of a carbon-hydrogen bond to afford a carbon radical and a hydrogen atom.
B) The first of the chain-propagating steps is rate-determining.
C) The reaction proceeds by way of a flatsp2 hybridized free radical.
D) Statements (The first of the chain-propagating steps is rate-determining) and (The reaction proceeds by way of a flatsp2 hybridized free radical) are both true.

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck



