Deck 20: Radical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/15
Play
Full screen (f)
Deck 20: Radical Reactions
1
Which of the following statements about bromination is true?
A) A single radical halogenation product predominates.
B) A mixture of products results.
C) The rate-determining step in bromination is exothermic.
D) Both radicals are formed.
A) A single radical halogenation product predominates.
B) A mixture of products results.
C) The rate-determining step in bromination is exothermic.
D) Both radicals are formed.
A single radical halogenation product predominates.
2
A possible reaction of ethane with chlorine is shown below.
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2]. Bond dissociation energies (kcal/mol): \begin{array}{|ll|} \hline \text { Bond } \mathrm{A}-\mathrm{B} & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3} \mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{Cl} & 81 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) +58 kcal/mol B) -5 kcal/mol C) None of the choices are correct. D) -28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_3e4c_862d_1f940fd58599_TBMG1035_00.jpg)
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2].
Bond dissociation energies (kcal/mol):
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2]. Bond dissociation energies (kcal/mol): \begin{array}{|ll|} \hline \text { Bond } \mathrm{A}-\mathrm{B} & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3} \mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{Cl} & 81 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) +58 kcal/mol B) -5 kcal/mol C) None of the choices are correct. D) -28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_3e4d_862d_1554f2c25501_TBMG1035_00.jpg)
A) +58 kcal/mol
B) -5 kcal/mol
C) None of the choices are correct.
D) -28 kcal/mol
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2]. Bond dissociation energies (kcal/mol): \begin{array}{|ll|} \hline \text { Bond } \mathrm{A}-\mathrm{B} & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3} \mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{Cl} & 81 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) +58 kcal/mol B) -5 kcal/mol C) None of the choices are correct. D) -28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_3e4c_862d_1f940fd58599_TBMG1035_00.jpg)
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2].
Bond dissociation energies (kcal/mol):
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [2]. Bond dissociation energies (kcal/mol): \begin{array}{|ll|} \hline \text { Bond } \mathrm{A}-\mathrm{B} & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3} \mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{Cl} & 81 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) +58 kcal/mol B) -5 kcal/mol C) None of the choices are correct. D) -28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_3e4d_862d_1554f2c25501_TBMG1035_00.jpg)
A) +58 kcal/mol
B) -5 kcal/mol
C) None of the choices are correct.
D) -28 kcal/mol
-5 kcal/mol
3
A possible reaction of ethane with chlorine is shown below.
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1]. Bond dissociation energies (kcal/mol): \begin{array}{|l|l|} \hline \text { Bond A-B } & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3}-\mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3}-\mathrm{Cl} & 84 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) -5 kcal/mol B) None of the choices are correct. C) -58 kcal/mol D)-28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_655f_862d_49af7dc5a63f_TBMG1035_00.jpg)
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1].
Bond dissociation energies (kcal/mol):
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1]. Bond dissociation energies (kcal/mol): \begin{array}{|l|l|} \hline \text { Bond A-B } & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3}-\mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3}-\mathrm{Cl} & 84 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) -5 kcal/mol B) None of the choices are correct. C) -58 kcal/mol D)-28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_6560_862d_d76708ee23ad_TBMG1035_00.jpg)
A) -5 kcal/mol
B) None of the choices are correct.
C) -58 kcal/mol
D)-28 kcal/mol
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1]. Bond dissociation energies (kcal/mol): \begin{array}{|l|l|} \hline \text { Bond A-B } & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3}-\mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3}-\mathrm{Cl} & 84 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) -5 kcal/mol B) None of the choices are correct. C) -58 kcal/mol D)-28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_655f_862d_49af7dc5a63f_TBMG1035_00.jpg)
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1].
Bond dissociation energies (kcal/mol):
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. Determine DH for step [1]. Bond dissociation energies (kcal/mol): \begin{array}{|l|l|} \hline \text { Bond A-B } & \Delta \mathrm{H} \\ \hline \mathrm{Cl}-\mathrm{Cl} & 58 \\ \mathrm{CH}_{3}-\mathrm{H} & 104 \\ \mathrm{CH}_{3} \mathrm{CH}_{2}-\mathrm{H} & 98 \\ \mathrm{CH}_{3}-\mathrm{CH}_{3} & 88 \\ \mathrm{CH}_{3}-\mathrm{Cl} & 84 \\ \mathrm{H}-\mathrm{Cl} & 103 \\ \hline \end{array} </strong> A) -5 kcal/mol B) None of the choices are correct. C) -58 kcal/mol D)-28 kcal/mol](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_6560_862d_d76708ee23ad_TBMG1035_00.jpg)
A) -5 kcal/mol
B) None of the choices are correct.
C) -58 kcal/mol
D)-28 kcal/mol
-58 kcal/mol
4
Which of the following statements about the stereochemistry of halogenation reactions is true?
A) An achiral starting material always gives an achiral product only.
B) The configuration at a stereogenic center of a product must change even if a reaction does not occur at a stereogenic center.
C) An achiral starting material always gives a racemic product only.
D) An achiral starting material always gives either an achiral or a racemic product.
A) An achiral starting material always gives an achiral product only.
B) The configuration at a stereogenic center of a product must change even if a reaction does not occur at a stereogenic center.
C) An achiral starting material always gives a racemic product only.
D) An achiral starting material always gives either an achiral or a racemic product.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about the propagation steps in the chlorination of ethane is true?
A) The first of the propagation steps is rate-determining because its transition state is at lower energy.
B) Radical chlorination consists of two propagation steps.
C) The second of the propagation steps is rate-determining because its transition state is at higher energy.
D) The energy diagram for the propagation steps has three energy barriers.
A) The first of the propagation steps is rate-determining because its transition state is at lower energy.
B) Radical chlorination consists of two propagation steps.
C) The second of the propagation steps is rate-determining because its transition state is at higher energy.
D) The energy diagram for the propagation steps has three energy barriers.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following are radical inhibitors?
A) Vitamin E
B) Both of these choices are correct.
C) BHT
D) None of these are correct.
A) Vitamin E
B) Both of these choices are correct.
C) BHT
D) None of these are correct.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
7
A possible reaction of ethane with chlorine is shown below.
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________.
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________. </strong> A) Only [3] B) Only [1] and [3] C) Only [2] and [3] D) Only [1] and [2]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_8c72_862d_87afbbcd9985_TBMG1035_00.jpg)
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________. </strong> A) Only [3] B) Only [1] and [3] C) Only [2] and [3] D) Only [1] and [2]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_8c73_862d_231e570c6d6c_TBMG1035_00.jpg)
A) Only [3]
B) Only [1] and [3]
C) Only [2] and [3]
D) Only [1] and [2]
This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________.
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________. </strong> A) Only [3] B) Only [1] and [3] C) Only [2] and [3] D) Only [1] and [2]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_8c72_862d_87afbbcd9985_TBMG1035_00.jpg)
![<strong>A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain propagating step(s) is (are) ________. </strong> A) Only [3] B) Only [1] and [3] C) Only [2] and [3] D) Only [1] and [2]](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfc_8c73_862d_231e570c6d6c_TBMG1035_00.jpg)
A) Only [3]
B) Only [1] and [3]
C) Only [2] and [3]
D) Only [1] and [2]

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about carbon radicals isnot true?
A) The unhybridizedp orbital in a carbon radical contains the unpaired electron.
B) A carbon radical issp2 hybridized.
C) Carbon radicals are classified as primary, secondary, tertiary, or quaternary.
D) The geometry of a carbon radical is trigonal planar.
A) The unhybridizedp orbital in a carbon radical contains the unpaired electron.
B) A carbon radical issp2 hybridized.
C) Carbon radicals are classified as primary, secondary, tertiary, or quaternary.
D) The geometry of a carbon radical is trigonal planar.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
9
What is the product of the following reaction? 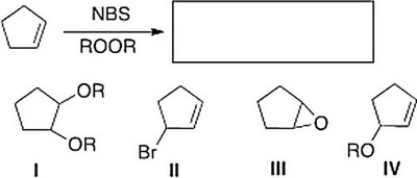
A) III
B) II
C) IV
D) I

A) III
B) II
C) IV
D) I

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is (are) true about free radical halogenation of alkanes?
A) The chain-initiating step involves cleavage of a carbon-hydrogen bond to afford a carbon radical and a hydrogen atom.
B) Statements (The first of the chain-propagating steps is rate-determining) and (The reaction proceeds by way of a flatsp2 hybridized free radical) are both true.
C) The reaction proceeds by way of a flatsp2 hybridized free radical.
D) The first of the chain-propagating steps is rate-determining.
A) The chain-initiating step involves cleavage of a carbon-hydrogen bond to afford a carbon radical and a hydrogen atom.
B) Statements (The first of the chain-propagating steps is rate-determining) and (The reaction proceeds by way of a flatsp2 hybridized free radical) are both true.
C) The reaction proceeds by way of a flatsp2 hybridized free radical.
D) The first of the chain-propagating steps is rate-determining.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements about radicals is true?
A) The higher the bond dissociation energy for a C-H bond, the more stable the resulting carbon radical.
B) Less stable radicals generally do not rearrange to more stable radicals.
C) The stability of a radical increases as the number of alkyl groups bonded to the radical carbon decreases.
D) Cleavage of a stronger bond forms the more stable radical.
A) The higher the bond dissociation energy for a C-H bond, the more stable the resulting carbon radical.
B) Less stable radicals generally do not rearrange to more stable radicals.
C) The stability of a radical increases as the number of alkyl groups bonded to the radical carbon decreases.
D) Cleavage of a stronger bond forms the more stable radical.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
12
What is the product of the following reaction? 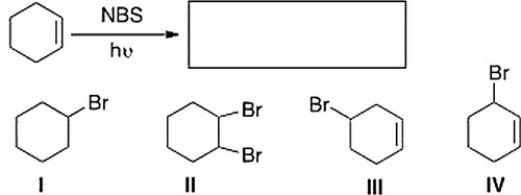
A) III
B) IV
C) II
D) I

A) III
B) IV
C) II
D) I

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
13
The proposed reaction between chlorofluorocarbons and ozone involves what type of mechanism?
A) Electrophilic aromatic substitution
B) Elimination mechanism
C) Substitution mechanism
D) Radical mechanism
A) Electrophilic aromatic substitution
B) Elimination mechanism
C) Substitution mechanism
D) Radical mechanism

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
14
The proposed reaction of the oxidation of unsaturated lipids involves what type of mechanism?
A) Elimination mechanism
B) Radical mechanism
C) Substitution mechanism
D) Electrophilic aromatic substitution
A) Elimination mechanism
B) Radical mechanism
C) Substitution mechanism
D) Electrophilic aromatic substitution

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
15
What is the product of the following reaction? 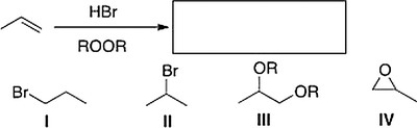
A) I
B) IV
C) III
D) II

A) I
B) IV
C) III
D) II

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck



