Deck 9: Alkenes Andalkynes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/24
Play
Full screen (f)
Deck 9: Alkenes Andalkynes
1
The hydroboration-oxidation to the following compound will result in: 
A) An alcohol
B) An alkyl halide
C) An aldehyde
D) A ketone

A) An alcohol
B) An alkyl halide
C) An aldehyde
D) A ketone
An aldehyde
2
Which of the following is a terpene? 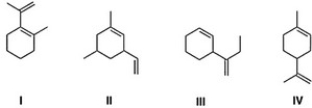
A) III
B) IV
C) I
D) II

A) III
B) IV
C) I
D) II
IV
3
Which of the following explains why 1-pentyne has a slightly higher boiling point than 1-pentene?
A) 1-Pentyne is linear while 1-pentene is trigonal planar.
B) 1-Pentyne has more carbons per hydrogen than 1-pentene.
C) The C-C triple bond in 1-pentyne is more polar than the C-C double bond in 1-pentene.
D) 1-Pentyne has more carbons than 1-pentene.
A) 1-Pentyne is linear while 1-pentene is trigonal planar.
B) 1-Pentyne has more carbons per hydrogen than 1-pentene.
C) The C-C triple bond in 1-pentyne is more polar than the C-C double bond in 1-pentene.
D) 1-Pentyne has more carbons than 1-pentene.
The C-C triple bond in 1-pentyne is more polar than the C-C double bond in 1-pentene.
4
Which alkene reacts fastest with HBr? 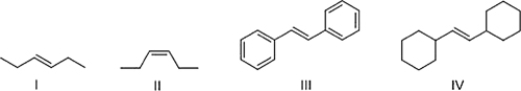
A) III
B) I
C) IV
D) II

A) III
B) I
C) IV
D) II

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
5
Which method would work the best in accomplishing the following transformation? ![<strong>Which method would work the best in accomplishing the following transformation? </strong> A) [1] HBr; [2] 2eq. NaNH<sub>2</sub> B) [1]BH<sub>3</sub>, THF; [2] H<sub>2</sub>O<sub>2</sub>, NaOH; [3] NaNH<sub>2</sub> C) [1] Br<sub>2</sub>, H<sub>2</sub>O; [2] NaNH<sub>2</sub> D) [1] Br<sub>2</sub>; [2] 2eq. NaNH<sub>2</sub>](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5d01_e308_862d_ebd10e3b8495_TBMG1035_00.jpg)
A) [1] HBr; [2] 2eq. NaNH2
B) [1]BH3, THF; [2] H2O2, NaOH; [3] NaNH2
C) [1] Br2, H2O; [2] NaNH2
D) [1] Br2; [2] 2eq. NaNH2
![<strong>Which method would work the best in accomplishing the following transformation? </strong> A) [1] HBr; [2] 2eq. NaNH<sub>2</sub> B) [1]BH<sub>3</sub>, THF; [2] H<sub>2</sub>O<sub>2</sub>, NaOH; [3] NaNH<sub>2</sub> C) [1] Br<sub>2</sub>, H<sub>2</sub>O; [2] NaNH<sub>2</sub> D) [1] Br<sub>2</sub>; [2] 2eq. NaNH<sub>2</sub>](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5d01_e308_862d_ebd10e3b8495_TBMG1035_00.jpg)
A) [1] HBr; [2] 2eq. NaNH2
B) [1]BH3, THF; [2] H2O2, NaOH; [3] NaNH2
C) [1] Br2, H2O; [2] NaNH2
D) [1] Br2; [2] 2eq. NaNH2

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
6
The addition of water in the presence of sulfuric acid to the following compound will result in: 
A) An alkyl halide
B) An aldehyde
C) A ketone
D) An alcohol

A) An alkyl halide
B) An aldehyde
C) A ketone
D) An alcohol

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
7
Give the IUPAC name for the following compound. 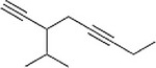
A) 3-sec-Butyl-1,4-nonadiyne
B) 3-Isopropyl-1,5-octadiyne
C) 3-sec-Butyl-1,4-octadiyne
D) 3-Isopropyl-1,5-nonadiyne

A) 3-sec-Butyl-1,4-nonadiyne
B) 3-Isopropyl-1,5-octadiyne
C) 3-sec-Butyl-1,4-octadiyne
D) 3-Isopropyl-1,5-nonadiyne

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the statements about the properties of the carbon-carbon double bond isnot true?
A) Trans alkenes are generally more stable than cis alkenes.
B) There is restricted rotation around the carbon-carbon double bond.
C) The stability of the carbon-carbon double bond increases as the number of substituent groups increases.
D) Whenever the two groups on each end of a carbon-carbon double bond are the same, two diastereomers are possible.
A) Trans alkenes are generally more stable than cis alkenes.
B) There is restricted rotation around the carbon-carbon double bond.
C) The stability of the carbon-carbon double bond increases as the number of substituent groups increases.
D) Whenever the two groups on each end of a carbon-carbon double bond are the same, two diastereomers are possible.

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
9
The addition of excess HBr to the following molecule will result in: 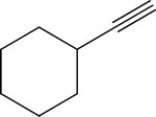
A) An alcohol
B) A geminal dihalide
C) A vicinal dihalide
D) An alkene

A) An alcohol
B) A geminal dihalide
C) A vicinal dihalide
D) An alkene

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
10
What is the major product of the following reaction? 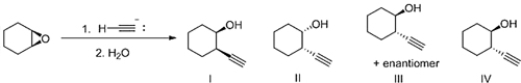
A) IV
B) III
C) II
D) I

A) IV
B) III
C) II
D) I

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
11
What is the major product of the following reaction? 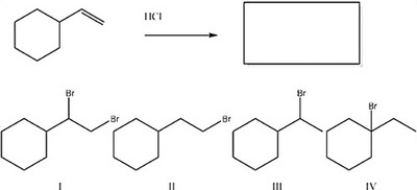
A) IV
B) II
C) III
D) I

A) IV
B) II
C) III
D) I

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
12
Give the IUPAC name for the following compound. 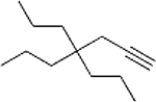
A) 4,4-Dipropyl-1-heptyne
B) 4,4-Diethyl-6-octyne
C) 4,4-Dipropyl-6-heptyne
D) 4,4-Diethyl-1-octyne

A) 4,4-Dipropyl-1-heptyne
B) 4,4-Diethyl-6-octyne
C) 4,4-Dipropyl-6-heptyne
D) 4,4-Diethyl-1-octyne

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
13
What is the product of the following reaction? 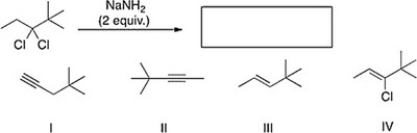
A) II
B) IV
C) III
D) I

A) II
B) IV
C) III
D) I

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements about the stereochemistry of electrophilic addition of HX to alkenes is true?
A) Hydrohalogenation occurs withsyn stereochemistry only.
B) Achiral starting materials yield an unequal mixture of two enantiomers.
C) Hydrohalogenation occurs withanti stereochemistry only.
D) Hydrohalogenation occurs withsyn andanti addition of HX.
A) Hydrohalogenation occurs withsyn stereochemistry only.
B) Achiral starting materials yield an unequal mixture of two enantiomers.
C) Hydrohalogenation occurs withanti stereochemistry only.
D) Hydrohalogenation occurs withsyn andanti addition of HX.

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
15
What is the major product of the following reaction? 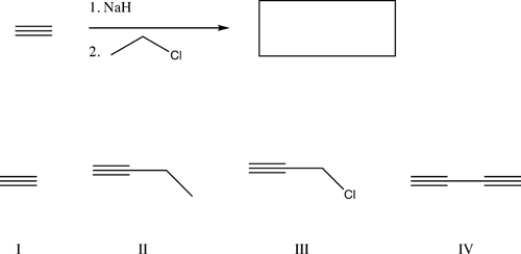
A) IV
B) I
C) II
D) III

A) IV
B) I
C) II
D) III

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
16
What is the product of the following reaction? 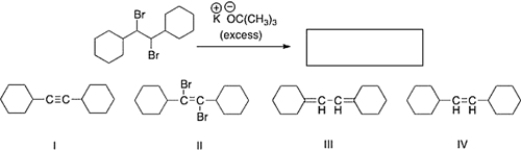
A) III
B) II
C) I
D) IV

A) III
B) II
C) I
D) IV

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds has the highest boiling point? 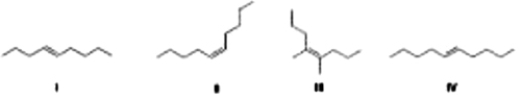
A) II
B) III
C) IV
D) I

A) II
B) III
C) IV
D) I

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
18
What is the major product of the following reaction? 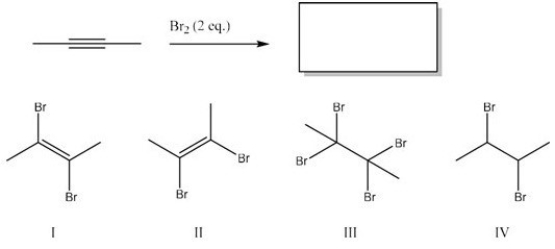
A) II
B) III
C) I
D) IV

A) II
B) III
C) I
D) IV

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
19
What is the major product of the following reaction? 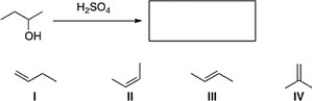
A) I
B) III
C) II
D) IV

A) I
B) III
C) II
D) IV

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
20
What is the major product of the following reaction? 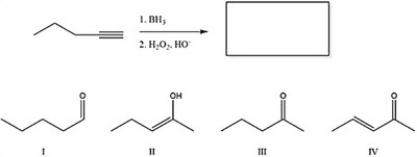
A) IV
B) III
C) I
D) II

A) IV
B) III
C) I
D) II

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
21
Predict the product of the following reaction. 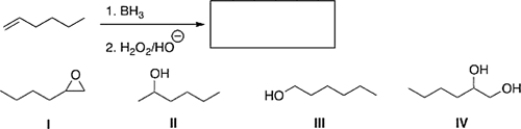
A) IV
B) II
C) I
D) III

A) IV
B) II
C) I
D) III

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements explain why HBr is a stronger acid than HF?
A) Br- is less stable than F- because Br- is less electronegative than F-.
B) Br- is less stable than F- because Br- is larger than F-.
C) Br- is more stable than F- because Br- is less electronegative than F-.
D) Br- is more stable than F- because Br- is larger than F-.
A) Br- is less stable than F- because Br- is less electronegative than F-.
B) Br- is less stable than F- because Br- is larger than F-.
C) Br- is more stable than F- because Br- is less electronegative than F-.
D) Br- is more stable than F- because Br- is larger than F-.

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is an acid commonly used it organic reactions?
A) HNO3
B) KOH
C) H2SO4
D) HCl
A) HNO3
B) KOH
C) H2SO4
D) HCl

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is an acid commonly used it organic reactions?
A) NaOH
B) NaNH2
C) HCl
D) KOH
A) NaOH
B) NaNH2
C) HCl
D) KOH

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck



