Deck 4: Proteins Iienzymes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/41
Play
Full screen (f)
Deck 4: Proteins Iienzymes
1
The ability for an enzyme to change its shape upon substrate binding represents the concept of _____.
A) lock and key
B) induced fit
C) proximity and orientation effects
D) covalent catalysis
E) none of the above
A) lock and key
B) induced fit
C) proximity and orientation effects
D) covalent catalysis
E) none of the above
induced fit
2
Chymotrypsin catalyzes the hydrolysis of a peptide bond and is therefore categorized as a(n) _____.
A) oxidoreductase
B) transferase
C) hydrolase
D) lyase
E) ligase
A) oxidoreductase
B) transferase
C) hydrolase
D) lyase
E) ligase
hydrolase
3
The highest point in a reaction coordinate diagram represents _____.
A) an intermediate of the reaction pathway
B) the reactants in an exergonic reaction
C) the products in an endergonic reaction
D) the transition state
E) the overall G for the reaction
A) an intermediate of the reaction pathway
B) the reactants in an exergonic reaction
C) the products in an endergonic reaction
D) the transition state
E) the overall G for the reaction
the transition state
4
If A B is a zero-order reaction, the rate is dependent on ______.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
5
A plot of velocity versus substrate concentration for a simple enzyme-catalyzed reaction produces a _____. This indicates that at some point, the enzyme is _____.
A) straight line; inhibited by product
B) hyperbolic curve; saturated with substrate
C) sigmoidal curve; inhibited by substrate
D) hyperbolic curve; activated by substrate
E) sigmoidal curve; saturated with substrate
A) straight line; inhibited by product
B) hyperbolic curve; saturated with substrate
C) sigmoidal curve; inhibited by substrate
D) hyperbolic curve; activated by substrate
E) sigmoidal curve; saturated with substrate

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
6
When a substrate and enzyme interact, the first chemical species formed is the _____.
A) enzyme-substrate complex
B) enzyme-transition state complex
C) enzyme-product complex
D) enzyme plus product
E) none of the above
A) enzyme-substrate complex
B) enzyme-transition state complex
C) enzyme-product complex
D) enzyme plus product
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following expresses the velocity for an enzyme-catalyzed reaction that obeys Michaelis-Menten kinetics?
A) v = k1[E][S]
B) v = k1[E][S] - k-1[ES]
C) v = k1[E][S] + k2[ES]
D) v = k2[ES]
E) v = k2[ES] - k-1[ES]
A) v = k1[E][S]
B) v = k1[E][S] - k-1[ES]
C) v = k1[E][S] + k2[ES]
D) v = k2[ES]
E) v = k2[ES] - k-1[ES]

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following must be true if the steady state assumption is to be used?
A) [E]T = [ES]
B) (k2 - k-1) / k1 = 1
C) k1[E][S] = k2[ES]
D) k1[E][S] = k2[ES] - k-1[ES]
E) d[ES] / dt = 0
A) [E]T = [ES]
B) (k2 - k-1) / k1 = 1
C) k1[E][S] = k2[ES]
D) k1[E][S] = k2[ES] - k-1[ES]
E) d[ES] / dt = 0

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
9
The Michaelis constant is defined as _____.
A) KM = k2 / k1
B) KM = (k2 + k-1) / k1
C) KM = (k2 - k-1) / k1
D) KM = (k2 + k1) / k-1
E) KM = (k2 - k1) / k-1
A) KM = k2 / k1
B) KM = (k2 + k-1) / k1
C) KM = (k2 - k-1) / k1
D) KM = (k2 + k1) / k-1
E) KM = (k2 - k1) / k-1

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
10
If an enzyme-catalyzed reaction has a velocity of 2 mM/min and a Vmax of 10 mM/min when the substrate concentration is 0.5 mM, what is the KM?
A) 0.2 mM
B) 0.5 mM
C) 1 mM
D) 2 mM
E) 5 mM
A) 0.2 mM
B) 0.5 mM
C) 1 mM
D) 2 mM
E) 5 mM

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
11
If an enzyme-catalyzed reaction with a KM of 3.5 mM has a velocity of 5 mM/min at a substrate concentration of 0.5 mM, what is the Vmax?
A) 0.625 mM/min
B) 15 mM/min
C) 17.5 mM/min
D) 35 mM/min
E) 40 mM/min
A) 0.625 mM/min
B) 15 mM/min
C) 17.5 mM/min
D) 35 mM/min
E) 40 mM/min

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
12
When [S] = KM, 0 = (_____) × (Vmax).
A) [S]
B) 0.75
C) 0.5
D) KM
E) kcat
A) [S]
B) 0.75
C) 0.5
D) KM
E) kcat

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
13
refer to the overall transformation shown in the following reaction: ![<strong>refer to the overall transformation shown in the following reaction: -Which of the following is (are) true?</strong> A) The [ES] will remain constant if k<sub>2</sub><sub> </sub>> k<sub>1</sub> and k<sub>-1</sub><sub> </sub>< k<sub>2</sub>. B) The reaction is zero order with respect to [S] if [S]>>[E] C) It describes a double displacement reaction D) All of the above are true. E) None of the above is true.](https://d2lvgg3v3hfg70.cloudfront.net/TB9579/11ee507e_826e_ba87_aff5_a78684470f7e_TB9579_00.jpg)
-Which of the following is (are) true?
A) The [ES] will remain constant if k2 > k1 and k-1 < k2.
B) The reaction is zero order with respect to [S] if [S]>>[E]
C) It describes a double displacement reaction
D) All of the above are true.
E) None of the above is true.
![<strong>refer to the overall transformation shown in the following reaction: -Which of the following is (are) true?</strong> A) The [ES] will remain constant if k<sub>2</sub><sub> </sub>> k<sub>1</sub> and k<sub>-1</sub><sub> </sub>< k<sub>2</sub>. B) The reaction is zero order with respect to [S] if [S]>>[E] C) It describes a double displacement reaction D) All of the above are true. E) None of the above is true.](https://d2lvgg3v3hfg70.cloudfront.net/TB9579/11ee507e_826e_ba87_aff5_a78684470f7e_TB9579_00.jpg)
-Which of the following is (are) true?
A) The [ES] will remain constant if k2 > k1 and k-1 < k2.
B) The reaction is zero order with respect to [S] if [S]>>[E]
C) It describes a double displacement reaction
D) All of the above are true.
E) None of the above is true.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
14
refer to the overall transformation shown in the following reaction: ![<strong>refer to the overall transformation shown in the following reaction: -For the reaction, the steady state assumption _____.</strong> A) implies that k<sub>1</sub><sub> </sub>= k<sub>-1</sub> B) implies that k<sub>-1</sub> and k<sub>2</sub> are such that the [ES] = k1[ES] C) [P]>>[E] D) [S] = [P] E) ES breakdown occurs at the same rate as ES formation](https://d2lvgg3v3hfg70.cloudfront.net/TB9579/11ee507e_826e_ba87_aff5_a78684470f7e_TB9579_00.jpg)
-For the reaction, the steady state assumption _____.
A) implies that k1 = k-1
B) implies that k-1 and k2 are such that the [ES] = k1[ES]
C) [P]>>[E]
D) [S] = [P]
E) ES breakdown occurs at the same rate as ES formation
![<strong>refer to the overall transformation shown in the following reaction: -For the reaction, the steady state assumption _____.</strong> A) implies that k<sub>1</sub><sub> </sub>= k<sub>-1</sub> B) implies that k<sub>-1</sub> and k<sub>2</sub> are such that the [ES] = k1[ES] C) [P]>>[E] D) [S] = [P] E) ES breakdown occurs at the same rate as ES formation](https://d2lvgg3v3hfg70.cloudfront.net/TB9579/11ee507e_826e_ba87_aff5_a78684470f7e_TB9579_00.jpg)
-For the reaction, the steady state assumption _____.
A) implies that k1 = k-1
B) implies that k-1 and k2 are such that the [ES] = k1[ES]
C) [P]>>[E]
D) [S] = [P]
E) ES breakdown occurs at the same rate as ES formation

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
15
A Lineweaver-Burk plot is a _____.
A) double reciprocal plot
B) Michaelis-Menten plot
C) sigmoidal plot
D) hyperbolic plot
E) logarithmic plot
A) double reciprocal plot
B) Michaelis-Menten plot
C) sigmoidal plot
D) hyperbolic plot
E) logarithmic plot

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
16
If a Lineweaver-Burk plot gave a line with an equation of y = 0.490 x + 0.059, what is the velocity at a substrate concentration of 5 mM? The original units for substrate were in mM and velocity in mM/s.
A) 0.288 mM/s
B) 0.399 mM/s
C) 2.51 mM/s
D) 6.37 mM/s
E) the velocity cannot be determined from these data
A) 0.288 mM/s
B) 0.399 mM/s
C) 2.51 mM/s
D) 6.37 mM/s
E) the velocity cannot be determined from these data

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
17
If a Lineweaver-Burk plot gave a line with an equation of y = 0.25 x + 0.34, what are the values of KM and Vmax if the substrate concentration is in mM and the velocity in mM/s?
A) 0.085 mM and 0.34 mM/s
B) 2.9 mM and 0.023 mM/s
C) 0.74 mM and 2.9 mM/s
D) 0.37 mM and 1.4 mM/s
E) 1.35 mM and .034 mM/s
A) 0.085 mM and 0.34 mM/s
B) 2.9 mM and 0.023 mM/s
C) 0.74 mM and 2.9 mM/s
D) 0.37 mM and 1.4 mM/s
E) 1.35 mM and .034 mM/s

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
18
Determine the KM and Vmax from the following graph. (Note: On the x-axis the minor tick mark spacing is 0.005; on the y-axis the minor tick mark spacing is 0.002) ![<strong>Determine the K<sub>M</sub> and V<sub>max</sub> from the following graph. (Note: On the x-axis the minor tick mark spacing is 0.005; on the y-axis the minor tick mark spacing is 0.002) </strong> A) K<sub>M</sub> = [0.006]; V<sub>max</sub> = 0.0075/s B) K<sub>M</sub> = [0.196]; V<sub>max</sub> = 0.0075/s C) K<sub>M</sub> = [165]; V<sub>max</sub> = 33/s D) K<sub>M</sub> = [33]; V<sub>max</sub> = 167/s E) K<sub>M</sub> = [270]; V<sub>max</sub> x = 68/s](https://d2lvgg3v3hfg70.cloudfront.net/TB9579/11ee507e_826e_e198_aff5_c176242095d8_TB9579_00.jpg)
A) KM = [0.006]; Vmax = 0.0075/s
B) KM = [0.196]; Vmax = 0.0075/s
C) KM = [165]; Vmax = 33/s
D) KM = [33]; Vmax = 167/s
E) KM = [270]; Vmax x = 68/s
![<strong>Determine the K<sub>M</sub> and V<sub>max</sub> from the following graph. (Note: On the x-axis the minor tick mark spacing is 0.005; on the y-axis the minor tick mark spacing is 0.002) </strong> A) K<sub>M</sub> = [0.006]; V<sub>max</sub> = 0.0075/s B) K<sub>M</sub> = [0.196]; V<sub>max</sub> = 0.0075/s C) K<sub>M</sub> = [165]; V<sub>max</sub> = 33/s D) K<sub>M</sub> = [33]; V<sub>max</sub> = 167/s E) K<sub>M</sub> = [270]; V<sub>max</sub> x = 68/s](https://d2lvgg3v3hfg70.cloudfront.net/TB9579/11ee507e_826e_e198_aff5_c176242095d8_TB9579_00.jpg)
A) KM = [0.006]; Vmax = 0.0075/s
B) KM = [0.196]; Vmax = 0.0075/s
C) KM = [165]; Vmax = 33/s
D) KM = [33]; Vmax = 167/s
E) KM = [270]; Vmax x = 68/s

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
19
Based on the figures below, which of the following expressions would be correct? 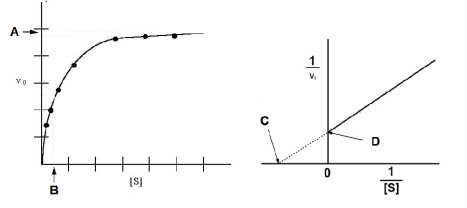
A) Vmax = 1/B
B) C = 1/Vmax
C) D = Vmax
D) D = 1/Vmax
E) A = 1/Vmax

A) Vmax = 1/B
B) C = 1/Vmax
C) D = Vmax
D) D = 1/Vmax
E) A = 1/Vmax

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
20
Based on the figure below, which of the following expressions would correctly define KM? 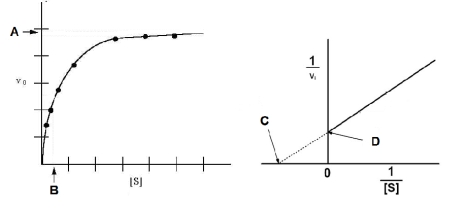
A) A = KM
B) KM = A/2
C) B = KM
D) C = -KM
E) D= 1/KM

A) A = KM
B) KM = A/2
C) B = KM
D) C = -KM
E) D= 1/KM

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
21
The catalytic constant, or kcat, is also known as the _____.
A) turnover number
B) saturation number
C) catalytic efficiency number
D) diffusion number
E) Menten number
A) turnover number
B) saturation number
C) catalytic efficiency number
D) diffusion number
E) Menten number

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following indicates that an enzyme has evolved to its most efficient form?
A) kcat is a large number
B) KM is a small number
C) KM is a large number
D) kcat/KM is a small number
E) kcat/KM is near the diffusion-controlled limit
A) kcat is a large number
B) KM is a small number
C) KM is a large number
D) kcat/KM is a small number
E) kcat/KM is near the diffusion-controlled limit

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
23
An extremely efficient enzyme has a _____ KM and a _____ kcat.
A) small; small
B) small; large
C) large; large
D) large; small
E) kcat and KM do nothing to predict the efficiency of an enzyme
A) small; small
B) small; large
C) large; large
D) large; small
E) kcat and KM do nothing to predict the efficiency of an enzyme

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
24
Some irreversible inhibitors are called _____ because they bind to the active site of the enzyme and begin the catalytic process, just like a normal substrate.
A) irreversible substrates
B) suicide substrates
C) noncompetitive substrates
D) ping pong substrates
E) allosteric substrates
A) irreversible substrates
B) suicide substrates
C) noncompetitive substrates
D) ping pong substrates
E) allosteric substrates

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
25
An inhibitor that binds to the active site only in the absence of the substrate and in a reversible fashion is a(n) _____.
A) allosteric inhibitor
B) suicide substrate
C) mixed inhibitor
D) noncompetitive inhibitor
E) competitive inhibitor
A) allosteric inhibitor
B) suicide substrate
C) mixed inhibitor
D) noncompetitive inhibitor
E) competitive inhibitor

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
26
What does the KI for a competitive inhibitor mean?
A) Higher KI values mean tighter binding to ES complex.
B) Lower KI values mean tighter binding to ES complex.
C) Higher KI values mean tighter binding to the enzyme.
D) Lower KI values mean tighter binding to the enzyme.
E) KI values tell nothing about inhibitor binding.
A) Higher KI values mean tighter binding to ES complex.
B) Lower KI values mean tighter binding to ES complex.
C) Higher KI values mean tighter binding to the enzyme.
D) Lower KI values mean tighter binding to the enzyme.
E) KI values tell nothing about inhibitor binding.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
27
A reversible inhibitor that binds to the enzyme only after one substrate has bound is a _____.
A) noncompetitive inhibitor
B) uncompetitive inhibitor
C) competitive inhibitor
D) allosteric inhibitor
E) suicide substrate
A) noncompetitive inhibitor
B) uncompetitive inhibitor
C) competitive inhibitor
D) allosteric inhibitor
E) suicide substrate

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following types of inhibition can be reversed by addition of more substrate?
A) noncompetitive inhibition
B) competitive inhibition
C) uncompetitive inhibition
D) irreversible inhibition
E) none of the above
A) noncompetitive inhibition
B) competitive inhibition
C) uncompetitive inhibition
D) irreversible inhibition
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
29
In ______, the inhibitor binds to a site involved in both substrate binding and catalysis.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
30
An enzyme that forms a covalent bond with its substrate during a reaction is considered to undergo _____.
A) acid-base catalysis
B) covalent catalysis
C) electrophilic catalysis
D) metal ion catalysis
E) none of the above
A) acid-base catalysis
B) covalent catalysis
C) electrophilic catalysis
D) metal ion catalysis
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following amino acids would most likely be found in the active site of an enzyme that uses acid-base catalysis?
A) Asn
B) Ser
C) Met
D) His
E) Trp
A) Asn
B) Ser
C) Met
D) His
E) Trp

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
32
Proton transfer from an acid, lowering the free energy of a reaction's transition state, is characteristic of _____.
A) electrostatic catalysis
B) nucleophilic catalysis
C) general base catalysis
D) general acid catalysis
E) concerted acid-base catalysis
A) electrostatic catalysis
B) nucleophilic catalysis
C) general base catalysis
D) general acid catalysis
E) concerted acid-base catalysis

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
33
What three amino acids are found in the catalytic triad of chymotrypsin?
A) Glu, His, Thr
B) Ser, Arg, Cys
C) Asp, His, Ser
D) Cys, Lys, Glu
E) Asn, His, Thr
A) Glu, His, Thr
B) Ser, Arg, Cys
C) Asp, His, Ser
D) Cys, Lys, Glu
E) Asn, His, Thr

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
34
What amino acid performs the nucleophilic attack during the chymotrypsin mechanism?
A) Ser
B) His
C) Lys
D) Cys
E) Thr
A) Ser
B) His
C) Lys
D) Cys
E) Thr

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
35
If the Asp in the chymotrypsin active site was mutated to another amino acid, which of the following would be considered an invisible mutation in that it is least likely to affect the function of the enzyme?
A) Asp Asn
B) Asp Glu
C) Asp His
D) Asp Ser
E) Asp Lys
A) Asp Asn
B) Asp Glu
C) Asp His
D) Asp Ser
E) Asp Lys

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
36
Chymotrypsin catalyzes the hydrolysis of peptide bonds adjacent to _____ residues in a peptide.
A) neutral polar
B) nonpolar
C) negatively charged
D) positively charged
E) all of the above because chymotrypsin has little substrate specificity
A) neutral polar
B) nonpolar
C) negatively charged
D) positively charged
E) all of the above because chymotrypsin has little substrate specificity

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
37
In the lysosome reaction, the carbohydrate substrate is in the _____ conformation providing a contribution of catalytic energy via the ____ distortion.
A) half-chair; electrostatic
B) chair; strain
C) boat; strain
D) boat; electrostatic
E) half-chair; strain
A) half-chair; electrostatic
B) chair; strain
C) boat; strain
D) boat; electrostatic
E) half-chair; strain

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
38
The molecule chymotrypsinogen is known as a(n) _____.
A) apoenzyme
B) holoenzyme
C) protease inhibitor
D) zymogen
E) none of the above
A) apoenzyme
B) holoenzyme
C) protease inhibitor
D) zymogen
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following represents a rapid and reversible mechanism to alter the activity of an enzyme?
A) synthesis of more enzyme to increase activity
B) degradation of enzyme to decrease activity
C) covalent attachment of a phosphate group to increase or decrease activity
D) movement of an enzyme from one cellular compartment to another
E) none of the above
A) synthesis of more enzyme to increase activity
B) degradation of enzyme to decrease activity
C) covalent attachment of a phosphate group to increase or decrease activity
D) movement of an enzyme from one cellular compartment to another
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
40
A common type of covalent modification of regulatory enzymes involves _____ of serine residues.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following statements regarding allosteric enzymes is true?
A) They are always oligomeric.
B) They are generally found at regulatory sites in metabolic pathways.
C) They are subject to regulation by both positive and negative effectors.
D) A plot of velocity versus [substrate] often yields a sigmoidal curve.
E) All of the above
A) They are always oligomeric.
B) They are generally found at regulatory sites in metabolic pathways.
C) They are subject to regulation by both positive and negative effectors.
D) A plot of velocity versus [substrate] often yields a sigmoidal curve.
E) All of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck



