Deck 20: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/143
Play
Full screen (f)
Deck 20: Electrochemistry
1
An electrolyte is
A)a solid that conducts electrical energy.
B)an inert electrode that conducts electrical energy.
C)a metal that conducts electrical energy through a solution.
D)a compound that conducts electricity either in solution or in the molten state.
E)a solvent that conducts electricity.
A)a solid that conducts electrical energy.
B)an inert electrode that conducts electrical energy.
C)a metal that conducts electrical energy through a solution.
D)a compound that conducts electricity either in solution or in the molten state.
E)a solvent that conducts electricity.
a compound that conducts electricity either in solution or in the molten state.
2
Anions
A)are charged ions that move toward the anode of a galvanic or electrolytic cell.
B)are charged ions that move toward the negative electrode of an electrolytic cell.
C)are charged ions that move toward the north pole of a magnetic field.
D)are positively charged ions that result from electrical discharge in a liquid solution.
E)are ions that attach themselves to any electrode to react chemically during electrolytic.
A)are charged ions that move toward the anode of a galvanic or electrolytic cell.
B)are charged ions that move toward the negative electrode of an electrolytic cell.
C)are charged ions that move toward the north pole of a magnetic field.
D)are positively charged ions that result from electrical discharge in a liquid solution.
E)are ions that attach themselves to any electrode to react chemically during electrolytic.
are charged ions that move toward the anode of a galvanic or electrolytic cell.
3
Cations
A)are negatively charged ions that result from electrical discharge in a liquid solution.
B)are charged ions that move toward the cathode of a galvanic or electrolytic cell.
C)are charged ions that move toward the positive electrode of an electrolytic cell.
D)are charged ions that move toward the south pole of a magnetic field.
E)are ions that attach themselves to any electrode to react chemically during electrolysis.
A)are negatively charged ions that result from electrical discharge in a liquid solution.
B)are charged ions that move toward the cathode of a galvanic or electrolytic cell.
C)are charged ions that move toward the positive electrode of an electrolytic cell.
D)are charged ions that move toward the south pole of a magnetic field.
E)are ions that attach themselves to any electrode to react chemically during electrolysis.
are charged ions that move toward the cathode of a galvanic or electrolytic cell.
4
A galvanic cell has two electrodes. Which statement is correct?
A)Oxidation takes place at the anode, which is positively charged.
B)Oxidation takes place at the anode, which is negatively charged.
C)Oxidation takes place at the cathode, which is positively charged.
D)Oxidation takes place at the cathode, which is negatively charged.
E)Oxidation take place at the dynode, which is uncharged.
A)Oxidation takes place at the anode, which is positively charged.
B)Oxidation takes place at the anode, which is negatively charged.
C)Oxidation takes place at the cathode, which is positively charged.
D)Oxidation takes place at the cathode, which is negatively charged.
E)Oxidation take place at the dynode, which is uncharged.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
5
A galvanic cell has two electrodes. Which statement is correct?
A)Reduction takes place at the anode, which is positively charged.
B)Reduction takes place at the anode, which is negatively charged.
C)Reduction takes place at the cathode, which is positively charged.
D)Reduction takes place at the cathode, which is negatively charged.
E)Reduction takes place at the dynode, which is uncharged.
A)Reduction takes place at the anode, which is positively charged.
B)Reduction takes place at the anode, which is negatively charged.
C)Reduction takes place at the cathode, which is positively charged.
D)Reduction takes place at the cathode, which is negatively charged.
E)Reduction takes place at the dynode, which is uncharged.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
6
A galvanic cell consists of a Cu(s)|Cu2+(aq)half-cell and a Zn(s)|Zn2+(aq)half-cell, connected by a salt bridge. Oxidation occurs in the zinc half-cell. The cell can be represented in standard notation as:
A)Cu(s)|Cu2+(aq)|Zn(s)|Zn2+(aq)
B)Zn(s)|Zn2+(aq)||Cu(s)|Cu2+(aq)
C)Cu2+(aq)|Cu(s)||Zn(s)|Zn2+(aq)
D)Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s)
E)Zn2+(aq)|Zn(s)||Cu(s)|Cu2+(aq)
A)Cu(s)|Cu2+(aq)|Zn(s)|Zn2+(aq)
B)Zn(s)|Zn2+(aq)||Cu(s)|Cu2+(aq)
C)Cu2+(aq)|Cu(s)||Zn(s)|Zn2+(aq)
D)Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s)
E)Zn2+(aq)|Zn(s)||Cu(s)|Cu2+(aq)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
7
A galvanic cell consists of an Ag(s)|Ag+(aq)half-cell and a Zn(s)|Zn2+(aq)half-cell connected by a salt bridge. Oxidation occurs in the zinc half-cell. The cell can be represented in standard notation as
A)Ag(s)|Ag+(aq)|Zn(s)|Zn2+(aq)
B)Zn(s)|Zn2+(aq)||Ag(s)|Ag+(aq)
C)Ag+(aq)|Ag(s)||Zn(s)|Zn2+(aq)
D)Zn2+(aq)|Zn(s)||Ag(s)|Ag+(aq)
E)Zn(s)|Zn2+(aq)||Ag+(aq)|Ag(s)
A)Ag(s)|Ag+(aq)|Zn(s)|Zn2+(aq)
B)Zn(s)|Zn2+(aq)||Ag(s)|Ag+(aq)
C)Ag+(aq)|Ag(s)||Zn(s)|Zn2+(aq)
D)Zn2+(aq)|Zn(s)||Ag(s)|Ag+(aq)
E)Zn(s)|Zn2+(aq)||Ag+(aq)|Ag(s)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
8
A galvanic cell consists of a Cd(s)|Cd2+(aq)half-cell and a Zn(s)|Zn2+(aq)half-cell connected by a salt bridge. Reduction occurs in the cadmium half-cell. The cell can be represented in standard notation as:
A)Cd(s)|Cd2+(aq)|Zn(s)|Zn2+(aq)
B)Zn(s)|Zn2+(aq)||Cd(s)|Cd2+(aq)
C)Zn(s)|Zn2+(aq)||Cd2+(aq)|Cd(s)
D)Zn2+(aq)|Zn(s)||Cd(s)|Cd2+(aq)
E)Cd2+(aq)|Cd(s)||Zn(s)|Zn2+(aq)
A)Cd(s)|Cd2+(aq)|Zn(s)|Zn2+(aq)
B)Zn(s)|Zn2+(aq)||Cd(s)|Cd2+(aq)
C)Zn(s)|Zn2+(aq)||Cd2+(aq)|Cd(s)
D)Zn2+(aq)|Zn(s)||Cd(s)|Cd2+(aq)
E)Cd2+(aq)|Cd(s)||Zn(s)|Zn2+(aq)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
9
A galvanic cell consists of a Cu(s)|Cu2+(aq)half-cell and a Cd(s)|Cd2+(aq)half-cell connected by a salt bridge. Oxidation occurs in the cadmium half-cell. The cell can be represented in standard notation as:
A)Cu(s)|Cu2+(aq)|Cd(s)|Cd2+(aq)
B)Cd(s)|Cd2+(aq)||Cu2+(aq)|Cu(s)
C)Cd2+(aq)|Cd(s)||Cu(s)|Cu2+(aq)
D)Cd(s)|Cd2+(aq)||Cu(s)|Cu2+(aq)
E)Cu2+(aq)|Cu(s)||Cd(s)|Cd2+(aq)
A)Cu(s)|Cu2+(aq)|Cd(s)|Cd2+(aq)
B)Cd(s)|Cd2+(aq)||Cu2+(aq)|Cu(s)
C)Cd2+(aq)|Cd(s)||Cu(s)|Cu2+(aq)
D)Cd(s)|Cd2+(aq)||Cu(s)|Cu2+(aq)
E)Cu2+(aq)|Cu(s)||Cd(s)|Cd2+(aq)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
10
A galvanic cell consists of an Ag(s)|Ag+(aq)half-cell and a Cu(s)|Cu2+(aq)half-cell connected by a salt bridge. The cell can be represented in standard notation as:
A)Ag(s)|Ag+(aq)|Cu(s)|Cu2+(aq)
B)Cu(s)|Cu2+(aq)||Ag(s)|Ag+(aq)
C)Ag+(aq)|Ag(s)||Cu(s)|Cu2+(aq)
D)Cu2+(aq)|Cu(s)||Ag(s)|Ag+(aq)
E)Cu(s)|Cu2+(aq)||Ag+(aq)|Ag(s)
A)Ag(s)|Ag+(aq)|Cu(s)|Cu2+(aq)
B)Cu(s)|Cu2+(aq)||Ag(s)|Ag+(aq)
C)Ag+(aq)|Ag(s)||Cu(s)|Cu2+(aq)
D)Cu2+(aq)|Cu(s)||Ag(s)|Ag+(aq)
E)Cu(s)|Cu2+(aq)||Ag+(aq)|Ag(s)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
11
A galvanic cell consists of an Ag(s)|Ag+(aq)half-cell and a Cu(s)|Cu2+(aq)half-cell connected by a salt bridge. The cell can be represented in standard notation as: Cu(s)|Cu2+(aq)||Ag+(aq)|Ag(s).Which species is being reduced?
A)Ag+(aq)
B)Cu2+(aq)
C)Ag(s)
D)Cu(s)
E)Cu+(aq)
A)Ag+(aq)
B)Cu2+(aq)
C)Ag(s)
D)Cu(s)
E)Cu+(aq)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
12
The electrode for which the standard reduction potential of 0.00 V is assigned uses the half-reaction:
A)Zn2+(aq)+ 2e- Zn(s)
Zn(s)
B)Cu2+(aq)+ 2e- Cu(s)
Cu(s)
C)Ag+(aq)+ e- Ag(s)
Ag(s)
D)2 H+(aq)+ 2e- H2(g)
H2(g)
E)2 NH4+(aq)+ 2e- H2(g)+ 2 NH3(g)
H2(g)+ 2 NH3(g)
A)Zn2+(aq)+ 2e-
 Zn(s)
Zn(s)B)Cu2+(aq)+ 2e-
 Cu(s)
Cu(s)C)Ag+(aq)+ e-
 Ag(s)
Ag(s)D)2 H+(aq)+ 2e-
 H2(g)
H2(g)E)2 NH4+(aq)+ 2e-
 H2(g)+ 2 NH3(g)
H2(g)+ 2 NH3(g)
Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
13
Using these metal ion/metal standard reduction potentials 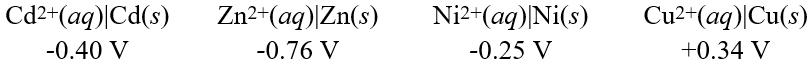 Calculate the standard cell potential for the cell whose reaction is:
Calculate the standard cell potential for the cell whose reaction is:
Cu2+(aq)+ Cd(s) Cd2+(aq)+ Cu(s)
A)+0.76 V
B)+0.06 V
C)-0.06 V
D)+0.74 V
E)+0.20 V
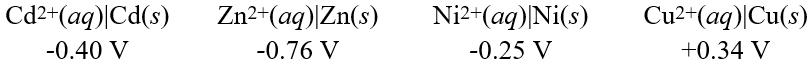 Calculate the standard cell potential for the cell whose reaction is:
Calculate the standard cell potential for the cell whose reaction is:Cu2+(aq)+ Cd(s) Cd2+(aq)+ Cu(s)
A)+0.76 V
B)+0.06 V
C)-0.06 V
D)+0.74 V
E)+0.20 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
14
Using these metal ion/metal standard reduction potentials 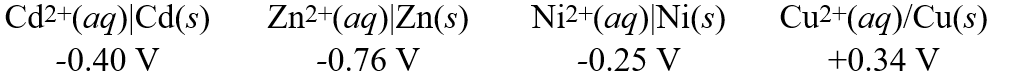
Calculate the standard cell potential for the cell whose reaction is:
Ni2+(aq)+ Zn(s) Zn2+(aq)+ Ni(s)
A)+0.51 V
B)-1.02 V
C)-1.01 V
D)+1.01 V
E)-0.51 V
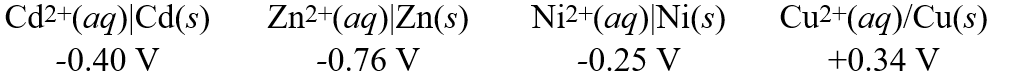
Calculate the standard cell potential for the cell whose reaction is:
Ni2+(aq)+ Zn(s) Zn2+(aq)+ Ni(s)
A)+0.51 V
B)-1.02 V
C)-1.01 V
D)+1.01 V
E)-0.51 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
15
Using these metal ion/metal standard reduction potentials 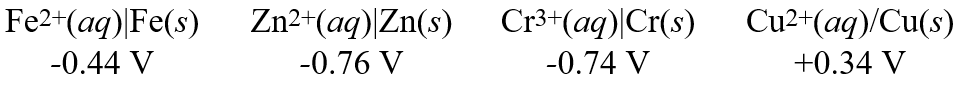
Calculate the standard cell potential for the cell whose reaction is:
Fe2+(aq)+ Cr(s) Fe(s)+ Cr3+(aq)
A)-0.30 V
B)-1.18 V
C)+0.30 V
D)+0.16 V
E)-0.16 V
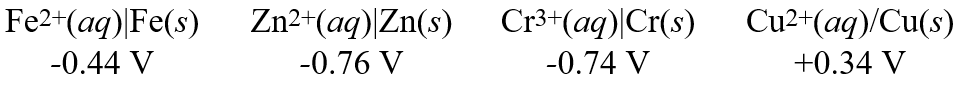
Calculate the standard cell potential for the cell whose reaction is:
Fe2+(aq)+ Cr(s) Fe(s)+ Cr3+(aq)
A)-0.30 V
B)-1.18 V
C)+0.30 V
D)+0.16 V
E)-0.16 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
16
Using these metal ion/metal reaction potentials: 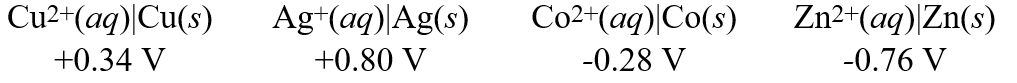 Calculate the standard cell potential for the cell whose reaction is:
Calculate the standard cell potential for the cell whose reaction is:
Co(s)+ Cu2+(aq) Co2+(aq)+ Cu(s)
A)-0.06 V
B)+0.06 V
C)-0.62 V
D)+0.62 V
E)+0.68 V
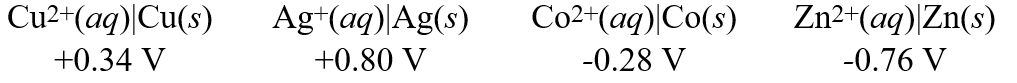 Calculate the standard cell potential for the cell whose reaction is:
Calculate the standard cell potential for the cell whose reaction is:Co(s)+ Cu2+(aq) Co2+(aq)+ Cu(s)
A)-0.06 V
B)+0.06 V
C)-0.62 V
D)+0.62 V
E)+0.68 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
17
Using the standard reduction potentials: 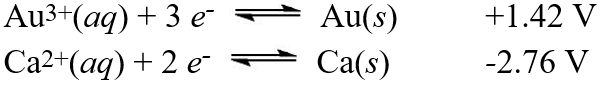 Calculate the value of E°cell for the reaction:
Calculate the value of E°cell for the reaction:
2Au(s)+ 3Ca2+(aq) 2Au3+(aq)+ 3Ca(s)
A)-1.43 V
B)+1.34 V
C)-4.18 V
D)+4.18 V
E)-1.34 V
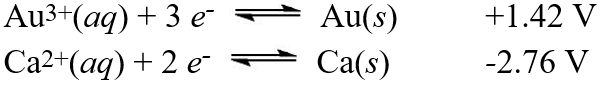 Calculate the value of E°cell for the reaction:
Calculate the value of E°cell for the reaction:2Au(s)+ 3Ca2+(aq) 2Au3+(aq)+ 3Ca(s)
A)-1.43 V
B)+1.34 V
C)-4.18 V
D)+4.18 V
E)-1.34 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
18
Using the standard reduction potentials:: 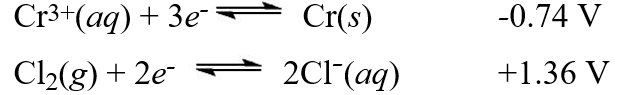 Calculate the value of E°cell for the cell with the reaction:2Cr(s)+ 3Cl2(g) 2Cr3+(aq)+ 6Cl-(aq)
Calculate the value of E°cell for the cell with the reaction:2Cr(s)+ 3Cl2(g) 2Cr3+(aq)+ 6Cl-(aq)
A)-0.96 V
B)+0.96 V
C)+2.10 V
D)-2.10 V
E)+0.98 V
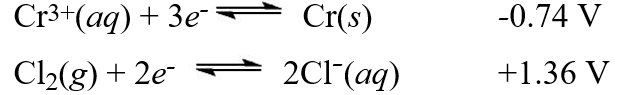 Calculate the value of E°cell for the cell with the reaction:2Cr(s)+ 3Cl2(g) 2Cr3+(aq)+ 6Cl-(aq)
Calculate the value of E°cell for the cell with the reaction:2Cr(s)+ 3Cl2(g) 2Cr3+(aq)+ 6Cl-(aq)A)-0.96 V
B)+0.96 V
C)+2.10 V
D)-2.10 V
E)+0.98 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
19
For the reaction, 2 Cr2+(aq)+ Cl2(g)  2 Cr3+(aq)+ 2 Cl-(aq), the value of E°cell is1.78 V. What is the value of E°cell for the following reaction?
2 Cr3+(aq)+ 2 Cl-(aq), the value of E°cell is1.78 V. What is the value of E°cell for the following reaction?
Cr3+(aq)+ Cl-(aq) Cr2+(aq)+ ½ Cl2(g)
A)-1.78 V
B)+0.89 V
C)+1.78 V
D)-0.89 V
E)-3.56 V
 2 Cr3+(aq)+ 2 Cl-(aq), the value of E°cell is1.78 V. What is the value of E°cell for the following reaction?
2 Cr3+(aq)+ 2 Cl-(aq), the value of E°cell is1.78 V. What is the value of E°cell for the following reaction?Cr3+(aq)+ Cl-(aq) Cr2+(aq)+ ½ Cl2(g)
A)-1.78 V
B)+0.89 V
C)+1.78 V
D)-0.89 V
E)-3.56 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
20
The cell described by the net reaction:2U(s)+ 3Cl2(g)  6 Cl-(aq)+ 2U3+(aq)has a standard cell potential of 3.16 Vs. Using the standard reduction potential value shown for:Cl2(g)+ 2 e-
6 Cl-(aq)+ 2U3+(aq)has a standard cell potential of 3.16 Vs. Using the standard reduction potential value shown for:Cl2(g)+ 2 e-  2 Cl-(aq)E° = +1.36 V determine the standard reduction potential of the U3+(aq)|U(s)half-cell
2 Cl-(aq)E° = +1.36 V determine the standard reduction potential of the U3+(aq)|U(s)half-cell
A)-1.80 V
B)+1.80 V
C)-1.96 V
D)-4.52 V
E)+4.52 V
 6 Cl-(aq)+ 2U3+(aq)has a standard cell potential of 3.16 Vs. Using the standard reduction potential value shown for:Cl2(g)+ 2 e-
6 Cl-(aq)+ 2U3+(aq)has a standard cell potential of 3.16 Vs. Using the standard reduction potential value shown for:Cl2(g)+ 2 e-  2 Cl-(aq)E° = +1.36 V determine the standard reduction potential of the U3+(aq)|U(s)half-cell
2 Cl-(aq)E° = +1.36 V determine the standard reduction potential of the U3+(aq)|U(s)half-cellA)-1.80 V
B)+1.80 V
C)-1.96 V
D)-4.52 V
E)+4.52 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
21
Consider this electrochemical cell:Pt | Pu3+(aq), Pu4+(aq)|| Cl2(g), Cl-(aq)| Pt Given that the standard cell potential is 0.35 V, what is the standard reduction potential E°(Pu4+/Pu3+)
A)2.37 V
B)1.71 V
C)1.01 V
D)-1.71 V
E)-1.01 V
A)2.37 V
B)1.71 V
C)1.01 V
D)-1.71 V
E)-1.01 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
22
The cell described by the reaction,2 Co3+(aq)+ 2 Cl-(aq)  2 Co2+(aq)+ Cl2(g)has a standard potential of 0.46 V. Using the standard reduction potential value shown for
2 Co2+(aq)+ Cl2(g)has a standard potential of 0.46 V. Using the standard reduction potential value shown for 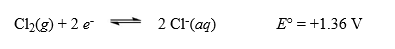 determine a value for the standard reduction potential of the cathode half-cell.
determine a value for the standard reduction potential of the cathode half-cell.
A)-0.90 V
B)+0.90 V
C)+0.91 V
D)-1.82 V
E)+1.82 V
 2 Co2+(aq)+ Cl2(g)has a standard potential of 0.46 V. Using the standard reduction potential value shown for
2 Co2+(aq)+ Cl2(g)has a standard potential of 0.46 V. Using the standard reduction potential value shown for 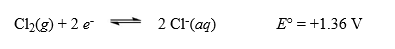 determine a value for the standard reduction potential of the cathode half-cell.
determine a value for the standard reduction potential of the cathode half-cell.A)-0.90 V
B)+0.90 V
C)+0.91 V
D)-1.82 V
E)+1.82 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
23
Consider these metal ion/metal standard reduction potentials 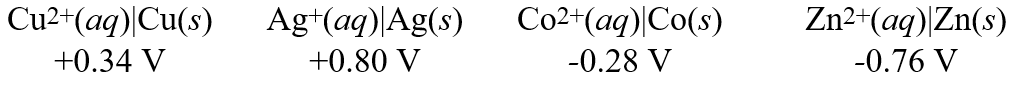 Based on the data above, which one of the species below is the best reducing agent?
Based on the data above, which one of the species below is the best reducing agent?
A)Co(s)
B)Zn(s)
C)Cu2+(aq)
D)Cu(s)
E)Ag(s)
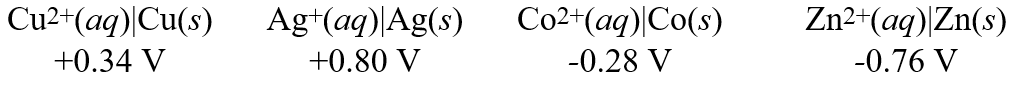 Based on the data above, which one of the species below is the best reducing agent?
Based on the data above, which one of the species below is the best reducing agent?A)Co(s)
B)Zn(s)
C)Cu2+(aq)
D)Cu(s)
E)Ag(s)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
24
Using the standard reduction potentials 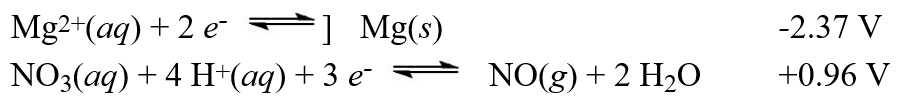 Calculate the value of E°cell for the cell with the reaction:
Calculate the value of E°cell for the cell with the reaction:
3 Mg(s)+ 2 NO3-(aq)+ 8 H+(aq) 3 Mg2+(aq)+ 2 NO(g)+ 4 H2O
A)+1.41 V
B)-1.41 V
C)+3.33 V
D)+8.46 V
E)-8.46 V
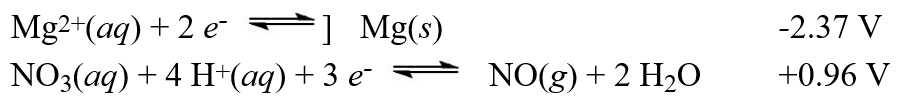 Calculate the value of E°cell for the cell with the reaction:
Calculate the value of E°cell for the cell with the reaction:3 Mg(s)+ 2 NO3-(aq)+ 8 H+(aq) 3 Mg2+(aq)+ 2 NO(g)+ 4 H2O
A)+1.41 V
B)-1.41 V
C)+3.33 V
D)+8.46 V
E)-8.46 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
25
Using the standard reduction potentials 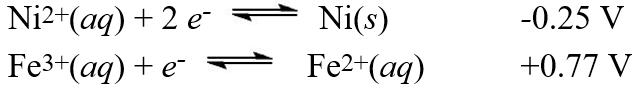 Calculate the value of E°cell for the cell with the following reaction.
Calculate the value of E°cell for the cell with the following reaction.
Ni2+(aq)+ 2 Fe2+(aq) Ni(s)+ 2 Fe3+(aq)
A)+0.52 V
B)-1.02 V
C)+2.81 V
D)+1.02 V
E)-2.81 V
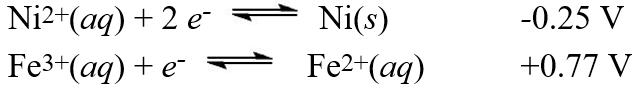 Calculate the value of E°cell for the cell with the following reaction.
Calculate the value of E°cell for the cell with the following reaction.Ni2+(aq)+ 2 Fe2+(aq) Ni(s)+ 2 Fe3+(aq)
A)+0.52 V
B)-1.02 V
C)+2.81 V
D)+1.02 V
E)-2.81 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
26
Consider these metal ion/metal standard reduction potentials 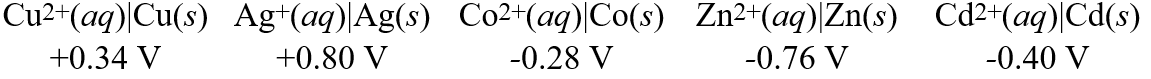 Based on the data above, which species is the best oxidizing agent?
Based on the data above, which species is the best oxidizing agent?
A)Co2+(aq)
B)Zn2+(aq)
C)Cu2+(aq)
D)Cd2+(aq)
E)Ag+(aq)
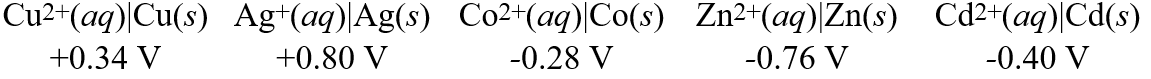 Based on the data above, which species is the best oxidizing agent?
Based on the data above, which species is the best oxidizing agent?A)Co2+(aq)
B)Zn2+(aq)
C)Cu2+(aq)
D)Cd2+(aq)
E)Ag+(aq)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
27
Consider these metal ion/metal standard reduction potentials 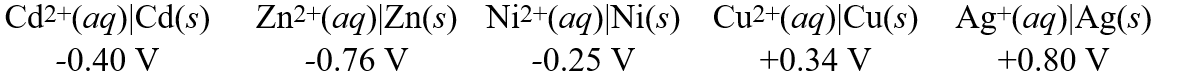 Based on the data above, which species is the best reducing agent?
Based on the data above, which species is the best reducing agent?
A)Cd(s)
B)Ag(s)
C)Ni(s)
D)Zn(s)
E)Cu(s)
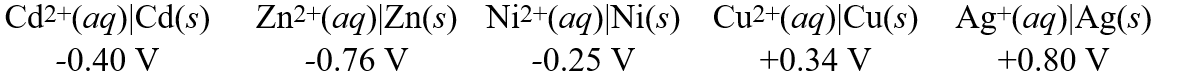 Based on the data above, which species is the best reducing agent?
Based on the data above, which species is the best reducing agent?A)Cd(s)
B)Ag(s)
C)Ni(s)
D)Zn(s)
E)Cu(s)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
28
Consider these metal ion/metal standard reduction potentials 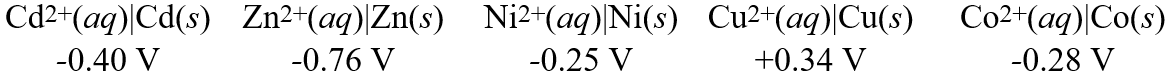 Based on the data above, which species is the best oxidizing agent?
Based on the data above, which species is the best oxidizing agent?
A)Cd2+(aq)
B)Zn2+(aq)
C)Co2+(aq)
D)Cu2+(aq)
E)Ni2+(aq)
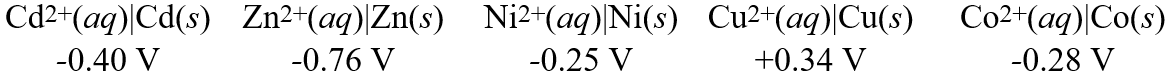 Based on the data above, which species is the best oxidizing agent?
Based on the data above, which species is the best oxidizing agent?A)Cd2+(aq)
B)Zn2+(aq)
C)Co2+(aq)
D)Cu2+(aq)
E)Ni2+(aq)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
29
Consider these metal ion/metal standard reduction potentials 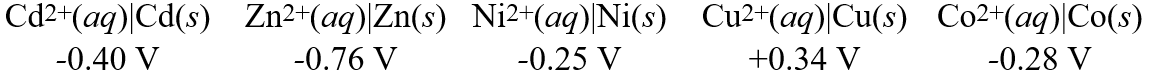 Based on the data above, which species is the best reducing agent?
Based on the data above, which species is the best reducing agent?
A)Co(s)
B)Cu(s)
C)Cd2+(aq)
D)Zn2+(aq)
E)Zn(s)
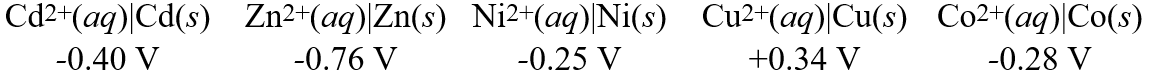 Based on the data above, which species is the best reducing agent?
Based on the data above, which species is the best reducing agent?A)Co(s)
B)Cu(s)
C)Cd2+(aq)
D)Zn2+(aq)
E)Zn(s)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
30
Consider these metal ion/metal standard reduction potentials 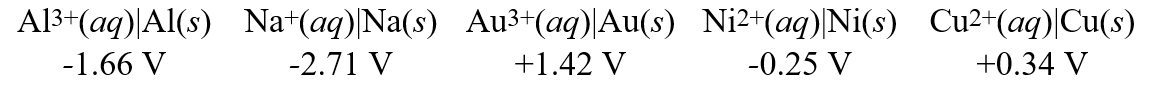 Based on the data above, which species is the best reducing agent?
Based on the data above, which species is the best reducing agent?
A)Ni(s)
B)Na(s)
C)Au(s)
D)Cu(s)
E)Al(s)
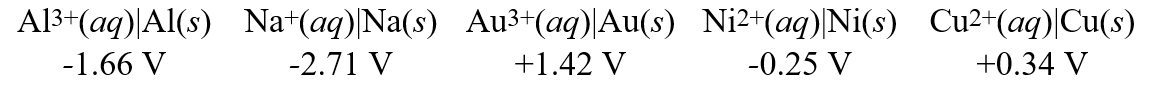 Based on the data above, which species is the best reducing agent?
Based on the data above, which species is the best reducing agent?A)Ni(s)
B)Na(s)
C)Au(s)
D)Cu(s)
E)Al(s)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
31
Which statement is true concerning a galvanic cell?
A)E° for the cell is always positive.
B)E° for the cell is always negative.
C)The standard reduction potential for the anode reaction is always positive.
D)The standard reduction potential for the anode reaction is always negative.
E)The standard reduction potential for the cathode reaction is always positive.
A)E° for the cell is always positive.
B)E° for the cell is always negative.
C)The standard reduction potential for the anode reaction is always positive.
D)The standard reduction potential for the anode reaction is always negative.
E)The standard reduction potential for the cathode reaction is always positive.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
32
A certain electrochemical cell has a cell potential of +0.34 V. Which of the following is a true statement about the electrochemical reaction?
A)The reaction favors the formation of reactants and would be considered an electrolytic cell.
B)The reaction favors the formation of reactants and would be considered a galvanic cell.
C)The reaction favors the formation of products and would be considered an electrolytic cell.
D)The reaction is at equilibrium and is a galvanic cell.
E)The reaction favors the formation of products and would be considered a galvanic cell.
A)The reaction favors the formation of reactants and would be considered an electrolytic cell.
B)The reaction favors the formation of reactants and would be considered a galvanic cell.
C)The reaction favors the formation of products and would be considered an electrolytic cell.
D)The reaction is at equilibrium and is a galvanic cell.
E)The reaction favors the formation of products and would be considered a galvanic cell.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
33
A unit of electrical energy is the
A)ampere.
B)coulomb.
C)joule.
D)volt.
E)watt.
A)ampere.
B)coulomb.
C)joule.
D)volt.
E)watt.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following reaction: 2Fe2+(aq)+ Cu2+ 2Fe3+(aq)+ Cu.When the ion concentrations change to the point where the reaction comes to equilibrium, what would be the cell voltage?
A)1.11 V
B)-0.43 V
C)0.0 V
D)0.43 V
E)0.78 V
A)1.11 V
B)-0.43 V
C)0.0 V
D)0.43 V
E)0.78 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
35
A unit of electrical charge used is the
A)ampere.
B)coulomb.
C)V.
D)joule.
E)watt.
A)ampere.
B)coulomb.
C)V.
D)joule.
E)watt.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
36
The Faraday constant is equal to the ________ on 1 mole of electrons.
A)capacitance
B)current
C)power
D)pressure
E)electrical charge
A)capacitance
B)current
C)power
D)pressure
E)electrical charge

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
37
One mole of electrical charge contains
A)4.184 joules.
B)3,600 coulombs.
C)23,060 joules.
D)96,485 coulombs.
E)3.47 × 108 coulombs.
A)4.184 joules.
B)3,600 coulombs.
C)23,060 joules.
D)96,485 coulombs.
E)3.47 × 108 coulombs.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
38
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 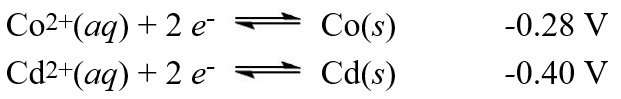 What is the standard free energy change for the cell reaction of this galvanic cell?
What is the standard free energy change for the cell reaction of this galvanic cell?
A)-12 kJ
B)+12 kJ
C)-23 kJ
D)+23 kJ
E)-46 kJ
 What is the standard free energy change for the cell reaction of this galvanic cell?
What is the standard free energy change for the cell reaction of this galvanic cell?A)-12 kJ
B)+12 kJ
C)-23 kJ
D)+23 kJ
E)-46 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
39
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 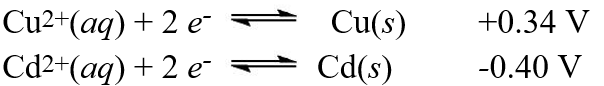 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
A)+12 kJ
B)-12 kJ
C)+143 kJ
D)-143 kJ
E)-71 kJ
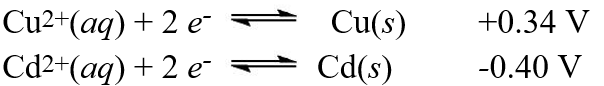 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?A)+12 kJ
B)-12 kJ
C)+143 kJ
D)-143 kJ
E)-71 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
40
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown:  What is the standard free energy change ( G°)for the cell reaction of this galvanic cell?
What is the standard free energy change ( G°)for the cell reaction of this galvanic cell?
A)69 kJ
B)+69 kJ
C)-224 kJ
D)+224 kJ
E)-35 kJ
 What is the standard free energy change ( G°)for the cell reaction of this galvanic cell?
What is the standard free energy change ( G°)for the cell reaction of this galvanic cell?A)69 kJ
B)+69 kJ
C)-224 kJ
D)+224 kJ
E)-35 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
41
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 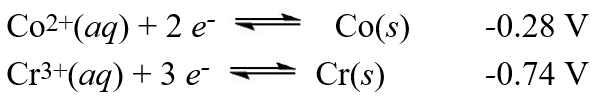 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
A)-88.8 kJ
B)-178 kJ
C)-266 kJ
D)-295 kJ
E)-590 kJ
 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?A)-88.8 kJ
B)-178 kJ
C)-266 kJ
D)-295 kJ
E)-590 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
42
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 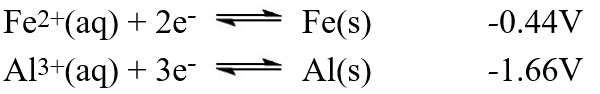 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
A)-806 kJ
B)-1.22 × 103 kJ
C)-706 kJ
D)-540 kJ
E)-600 kJ
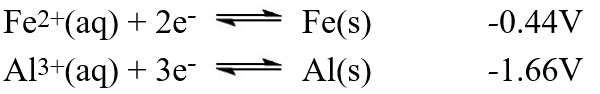 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?A)-806 kJ
B)-1.22 × 103 kJ
C)-706 kJ
D)-540 kJ
E)-600 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
43
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 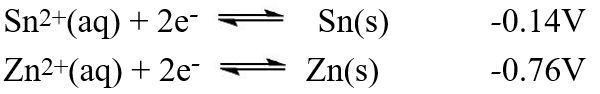 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
A)-2.22 × 102 kJ
B)-3.14 × 102 kJ
C)-1.74 × 102 kJ
D)-6.02 × 102 kJ
E)-1.20 × 102 kJ
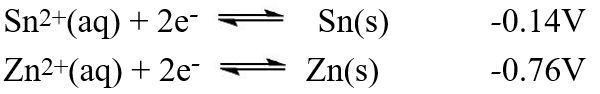 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?A)-2.22 × 102 kJ
B)-3.14 × 102 kJ
C)-1.74 × 102 kJ
D)-6.02 × 102 kJ
E)-1.20 × 102 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
44
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 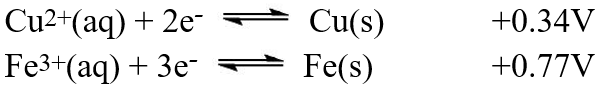 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
A)-2.49 × 102 kJ
B)-3.21 × 102 kJ
C)-6.43 × 102 kJ
D)-5.32 × 102 kJ
E)-4.31 × 102 kJ
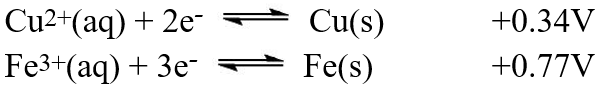 What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°)change for the cell reaction of this galvanic cell?A)-2.49 × 102 kJ
B)-3.21 × 102 kJ
C)-6.43 × 102 kJ
D)-5.32 × 102 kJ
E)-4.31 × 102 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
45
Using the standard reduction potentials 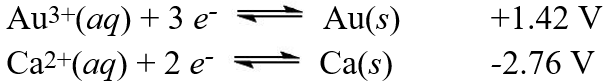 Calculate the standard free energy ( G°)change for the cell reaction:
Calculate the standard free energy ( G°)change for the cell reaction:
2 Au(s)+ 3 Ca2+(aq) 2 Au3+(aq)+ 3 Ca(s)
A)2420 kJ
B)388 kJ
C)-766 kJ
D)766 kJ
E)-1210 kJ
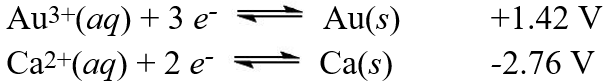 Calculate the standard free energy ( G°)change for the cell reaction:
Calculate the standard free energy ( G°)change for the cell reaction:2 Au(s)+ 3 Ca2+(aq) 2 Au3+(aq)+ 3 Ca(s)
A)2420 kJ
B)388 kJ
C)-766 kJ
D)766 kJ
E)-1210 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
46
Using these metal ion/metal standard reduction potentials 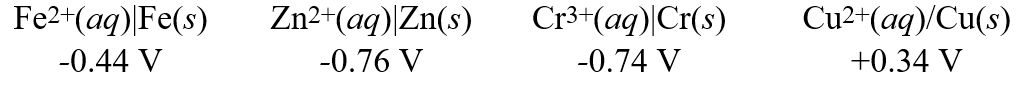 Calculate the standard free energy ( G°)change for the cell reaction:Fe2+(aq)+ Cr(s) Fe(s)+ Cr3+(aq)
Calculate the standard free energy ( G°)change for the cell reaction:Fe2+(aq)+ Cr(s) Fe(s)+ Cr3+(aq)
A)-92.6 kJ
B)-86.8 kJ
C)683.1 kJ
D)-57.9 kJ
E)-173.7 kJ
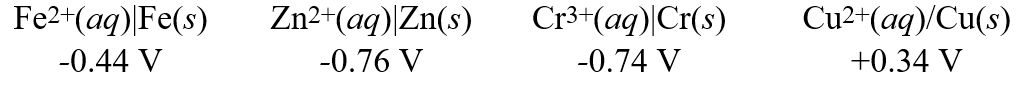 Calculate the standard free energy ( G°)change for the cell reaction:Fe2+(aq)+ Cr(s) Fe(s)+ Cr3+(aq)
Calculate the standard free energy ( G°)change for the cell reaction:Fe2+(aq)+ Cr(s) Fe(s)+ Cr3+(aq)A)-92.6 kJ
B)-86.8 kJ
C)683.1 kJ
D)-57.9 kJ
E)-173.7 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
47
Using the reduction potentials given, calculate the equilibrium constant, K, at 25°C for the reaction, 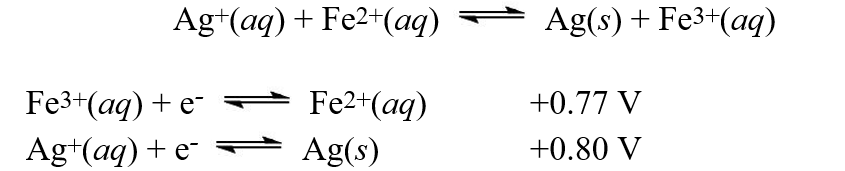
A)1.66
B)6.4
C)3.2
D)6.1 × 10-4
E)1.6 × 104
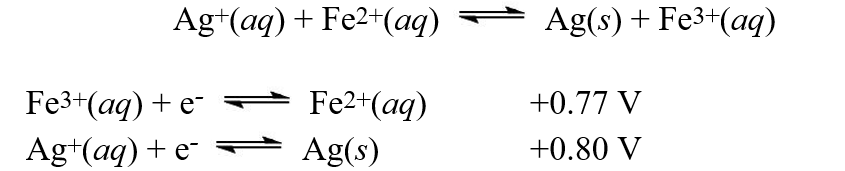
A)1.66
B)6.4
C)3.2
D)6.1 × 10-4
E)1.6 × 104

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
48
The equilibrium constant, Kc, was found to be 1.2 × 103 at 25°C for the reaction,2X(s)+ Cu2+(aq)  2X+(aq)+ Cu(s)Using the following reduction potential for copper, what is the reduction potential for the other half reaction involving the substance X?
2X+(aq)+ Cu(s)Using the following reduction potential for copper, what is the reduction potential for the other half reaction involving the substance X? 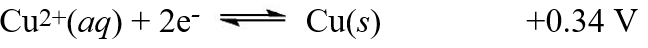
A)-0.16 V
B)0.091 V
C)0.52 V
D)0.18 V
E)-0.25 V
 2X+(aq)+ Cu(s)Using the following reduction potential for copper, what is the reduction potential for the other half reaction involving the substance X?
2X+(aq)+ Cu(s)Using the following reduction potential for copper, what is the reduction potential for the other half reaction involving the substance X? 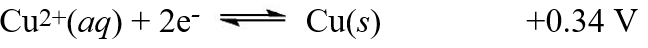
A)-0.16 V
B)0.091 V
C)0.52 V
D)0.18 V
E)-0.25 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
49
Using the reduction potentials given, calculate the equilibrium constant, K, at 25°C for the reaction, 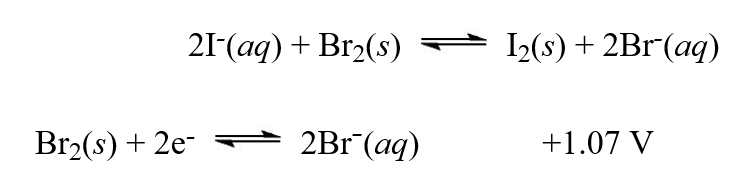
A)8.5 × 1017
B)6.8
C)2.4 × 104
D)1.02
E)1.2 × 10-18
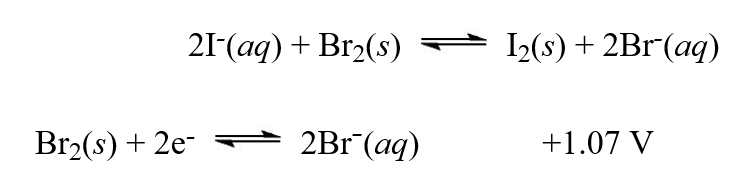
A)8.5 × 1017
B)6.8
C)2.4 × 104
D)1.02
E)1.2 × 10-18

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
50
The equilibrium constant, Kc, was found to be 2.4 × 108 at 25°C for the following reaction,2X(s)+ 3Y2+(aq)  2X3+(aq)+ 3Y(s)Using this information, what is the standard reduction potential for this reaction?
2X3+(aq)+ 3Y(s)Using this information, what is the standard reduction potential for this reaction?
A)0.25 V
B)0.083 V
C)0.17 V
D)0.50 V
E)0.21 V
 2X3+(aq)+ 3Y(s)Using this information, what is the standard reduction potential for this reaction?
2X3+(aq)+ 3Y(s)Using this information, what is the standard reduction potential for this reaction?A)0.25 V
B)0.083 V
C)0.17 V
D)0.50 V
E)0.21 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
51
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: ![<strong>A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: The actual concentrations are: [Co<sup>2+</sup>(aq)] = 0.00100 M, [Cd<sup>2+</sup>] = 0.100 M. What is the potential of this galvanic cell? Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+0.18 V B)+0.12 V C)+0.24 V D)+0.060 V E)+0.68 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_06a0_bf7f_771f8ecd9b3c_TBW1039_00.jpg) The actual concentrations are: [Co2+(aq)] = 0.00100 M, [Cd2+] = 0.100 M. What is the potential of this galvanic cell? Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations are: [Co2+(aq)] = 0.00100 M, [Cd2+] = 0.100 M. What is the potential of this galvanic cell? Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
A)+0.18 V
B)+0.12 V
C)+0.24 V
D)+0.060 V
E)+0.68 V
![<strong>A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: The actual concentrations are: [Co<sup>2+</sup>(aq)] = 0.00100 M, [Cd<sup>2+</sup>] = 0.100 M. What is the potential of this galvanic cell? Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+0.18 V B)+0.12 V C)+0.24 V D)+0.060 V E)+0.68 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_06a0_bf7f_771f8ecd9b3c_TBW1039_00.jpg) The actual concentrations are: [Co2+(aq)] = 0.00100 M, [Cd2+] = 0.100 M. What is the potential of this galvanic cell? Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations are: [Co2+(aq)] = 0.00100 M, [Cd2+] = 0.100 M. What is the potential of this galvanic cell? Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.A)+0.18 V
B)+0.12 V
C)+0.24 V
D)+0.060 V
E)+0.68 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
52
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: = 0.100 M, [Cd<sup>2+</sup>] = 0.0100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+0.06 V B)+0.09 V C)+0.15 V D)+0.18 V E)+0.24 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_06a1_bf7f_17587bd2f1c0_TBW1039_00.jpg) The actual concentrations in the cell are: [Co2+](aq)= 0.100 M, [Cd2+] = 0.0100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations in the cell are: [Co2+](aq)= 0.100 M, [Cd2+] = 0.0100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
A)+0.06 V
B)+0.09 V
C)+0.15 V
D)+0.18 V
E)+0.24 V
= 0.100 M, [Cd<sup>2+</sup>] = 0.0100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+0.06 V B)+0.09 V C)+0.15 V D)+0.18 V E)+0.24 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_06a1_bf7f_17587bd2f1c0_TBW1039_00.jpg) The actual concentrations in the cell are: [Co2+](aq)= 0.100 M, [Cd2+] = 0.0100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations in the cell are: [Co2+](aq)= 0.100 M, [Cd2+] = 0.0100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.A)+0.06 V
B)+0.09 V
C)+0.15 V
D)+0.18 V
E)+0.24 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
53
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: = 0.0100 M, [Cr<sup>3+</sup>] = 0.00100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+0.40 V B)+0.46 V C)+0.52 V D)+0.54 V E)+1.02 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_2db2_bf7f_e9be3ee2b444_TBW1039_00.jpg) The actual concentrations in the cell are: [Co2+](aq)= 0.0100 M, [Cr3+] = 0.00100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations in the cell are: [Co2+](aq)= 0.0100 M, [Cr3+] = 0.00100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
A)+0.40 V
B)+0.46 V
C)+0.52 V
D)+0.54 V
E)+1.02 V
= 0.0100 M, [Cr<sup>3+</sup>] = 0.00100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+0.40 V B)+0.46 V C)+0.52 V D)+0.54 V E)+1.02 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_2db2_bf7f_e9be3ee2b444_TBW1039_00.jpg) The actual concentrations in the cell are: [Co2+](aq)= 0.0100 M, [Cr3+] = 0.00100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations in the cell are: [Co2+](aq)= 0.0100 M, [Cr3+] = 0.00100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.A)+0.40 V
B)+0.46 V
C)+0.52 V
D)+0.54 V
E)+1.02 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
54
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: ![<strong>A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: The actual concentrations in the cell are: [Co<sup>2+</sup>] = 0.00100 M, [Cr<sup>3+</sup>]= 0.100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+0.33 V B)+0.39 V C)+0.45 V D)+0.94 V E)+1.61 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_2db3_bf7f_131f1dfd421e_TBW1039_00.jpg) The actual concentrations in the cell are: [Co2+] = 0.00100 M, [Cr3+]= 0.100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations in the cell are: [Co2+] = 0.00100 M, [Cr3+]= 0.100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
A)+0.33 V
B)+0.39 V
C)+0.45 V
D)+0.94 V
E)+1.61 V
![<strong>A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: The actual concentrations in the cell are: [Co<sup>2+</sup>] = 0.00100 M, [Cr<sup>3+</sup>]= 0.100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+0.33 V B)+0.39 V C)+0.45 V D)+0.94 V E)+1.61 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_2db3_bf7f_131f1dfd421e_TBW1039_00.jpg) The actual concentrations in the cell are: [Co2+] = 0.00100 M, [Cr3+]= 0.100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations in the cell are: [Co2+] = 0.00100 M, [Cr3+]= 0.100 M. What is the potential of this galvanic cell?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.A)+0.33 V
B)+0.39 V
C)+0.45 V
D)+0.94 V
E)+1.61 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
55
A galvanic cell is composed of these two half-cells: ![<strong>A galvanic cell is composed of these two half-cells: The actual concentrations in the cell are: [Cu<sup>2+</sup>] = 0.00350 M, [Cr<sup>3+</sup>] = 0.360 M. What is the potential of this galvanic cell at 25°C?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+1.06 V B)-0.16 V C)+1.02 V D)+1.14 V E)+1.98 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_2db4_bf7f_233becec4615_TBW1039_00.jpg) The actual concentrations in the cell are: [Cu2+] = 0.00350 M, [Cr3+] = 0.360 M. What is the potential of this galvanic cell at 25°C?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations in the cell are: [Cu2+] = 0.00350 M, [Cr3+] = 0.360 M. What is the potential of this galvanic cell at 25°C?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
A)+1.06 V
B)-0.16 V
C)+1.02 V
D)+1.14 V
E)+1.98 V
![<strong>A galvanic cell is composed of these two half-cells: The actual concentrations in the cell are: [Cu<sup>2+</sup>] = 0.00350 M, [Cr<sup>3+</sup>] = 0.360 M. What is the potential of this galvanic cell at 25°C?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.</strong> A)+1.06 V B)-0.16 V C)+1.02 V D)+1.14 V E)+1.98 V](https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/11ee6e7b_ba5c_2db4_bf7f_233becec4615_TBW1039_00.jpg) The actual concentrations in the cell are: [Cu2+] = 0.00350 M, [Cr3+] = 0.360 M. What is the potential of this galvanic cell at 25°C?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
The actual concentrations in the cell are: [Cu2+] = 0.00350 M, [Cr3+] = 0.360 M. What is the potential of this galvanic cell at 25°C?Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.A)+1.06 V
B)-0.16 V
C)+1.02 V
D)+1.14 V
E)+1.98 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
56
The standard reduction potentials of Cu2+(aq)|Cu(s)and Ag+(aq)|Ag(s)are +0.34 and+0.80 V, respectively. Determine the value of the actual cell potential, Ecell, (in V)for the following cell at 25.0 °C.
Cu(s)|Cu2+(0.250 M)||Ag+(0.0010 M)|Ag(s)Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
A)+0.30 V
B)+0.14 V
C)+0.62 V
D)+0.78 V
E)+0.39 V
Cu(s)|Cu2+(0.250 M)||Ag+(0.0010 M)|Ag(s)Hint: First calculate E°, then apply the solution concentrations of the galvanic cell using the Nernst equation.
A)+0.30 V
B)+0.14 V
C)+0.62 V
D)+0.78 V
E)+0.39 V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
57
Fuel cells are different from other traditional batteries because
A)they require a constant supply of reactants to produce voltage.
B)they are only used in space.
C)they have a solid medium.
D)they require voltage to work.
E)they utilize heat from combustion of gases.
A)they require a constant supply of reactants to produce voltage.
B)they are only used in space.
C)they have a solid medium.
D)they require voltage to work.
E)they utilize heat from combustion of gases.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
58
When fused (molten)sodium chloride is electrolyzed what occurs?
A)Gaseous chlorine is formed at the cathode.
B)Hydrogen gas is formed at the cathode.
C)Liquid sodium is formed at the cathode.
D)Liquid chlorine is formed at the anode.
E)Solid sodium is formed at the anode.
A)Gaseous chlorine is formed at the cathode.
B)Hydrogen gas is formed at the cathode.
C)Liquid sodium is formed at the cathode.
D)Liquid chlorine is formed at the anode.
E)Solid sodium is formed at the anode.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
59
In doping semiconductor materials,
A)the energy gap between the valence band and the conduction band is completely removed and a covalent bond is formed.
B)impurities are added that either provide extra electrons, or 'holes' for electrons to move through.
C)a large flow of electricity is added to the material kicking electrons out of the material and creating 'holes'.
D)the material is destroyed using an acid.
E)the material is dissolved in an organic solvent.
A)the energy gap between the valence band and the conduction band is completely removed and a covalent bond is formed.
B)impurities are added that either provide extra electrons, or 'holes' for electrons to move through.
C)a large flow of electricity is added to the material kicking electrons out of the material and creating 'holes'.
D)the material is destroyed using an acid.
E)the material is dissolved in an organic solvent.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
60
When an aqueous solution of AgNO3 is electrolyzed, a gas is formed at the anode. The gas is
A)dinitrogen tetroxide.
B)hydrogen.
C)mononitrogen monoxide.
D)nitrogen dioxide.
E)oxygen.
A)dinitrogen tetroxide.
B)hydrogen.
C)mononitrogen monoxide.
D)nitrogen dioxide.
E)oxygen.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
61
Electrolysis is
A)the splitting of atomic nuclei by electrical energy.
B)the splitting of atoms by electrical energy.
C)the passage of electrical energy through a split-field armature.
D)the chemical reaction which results when electrical energy is passed through a liquid electrolyte.
E)the chemical reaction which results when electrical energy is passed through a metallic liquid.
A)the splitting of atomic nuclei by electrical energy.
B)the splitting of atoms by electrical energy.
C)the passage of electrical energy through a split-field armature.
D)the chemical reaction which results when electrical energy is passed through a liquid electrolyte.
E)the chemical reaction which results when electrical energy is passed through a metallic liquid.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
62
Which statement below is true?
A)Electrolysis cells generate alternating current when their terminals are reversed.
B)Electrolysis was discovered by Lewis Latimer.
C)Galvanic cells generate electrical energy rather than consuming it.
D)Galvanic cells were invented by Thomas Edison.
E)The Laws of Electrolysis were discovered by Alberta Nernst.
A)Electrolysis cells generate alternating current when their terminals are reversed.
B)Electrolysis was discovered by Lewis Latimer.
C)Galvanic cells generate electrical energy rather than consuming it.
D)Galvanic cells were invented by Thomas Edison.
E)The Laws of Electrolysis were discovered by Alberta Nernst.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
63
When an aqueous solution of sodium chloride is electrolyzed, hydrogen gas is evolved at the cathode. The solution near the cathode becomes
A)acidic.
B)basic.
C)bubbly.
D)colored.
E)viscous.
A)acidic.
B)basic.
C)bubbly.
D)colored.
E)viscous.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
64
When an aqueous solution of sodium sulfate is electrolyzed, a gas is evolved at the anode. The solution near the anode becomes
A)acidic.
B)basic.
C)bubbly.
D)colored.
E)viscous.
A)acidic.
B)basic.
C)bubbly.
D)colored.
E)viscous.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
65
An electrolytic cell has two electrodes. Which statement is correct?
A)Oxidation takes place at the anode, which is positively charged.
B)Oxidation takes place at the anode, which is negatively charged.
C)Oxidation takes place at the cathode, which is positively charged.
D)Oxidation takes place at the cathode, which is negatively charged.
E)Oxidation take place at the dynode, which is uncharged.
A)Oxidation takes place at the anode, which is positively charged.
B)Oxidation takes place at the anode, which is negatively charged.
C)Oxidation takes place at the cathode, which is positively charged.
D)Oxidation takes place at the cathode, which is negatively charged.
E)Oxidation take place at the dynode, which is uncharged.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
66
An electrolysis cell has two electrodes. Which statement is correct?
A)Reduction takes place at the anode, which is positively charged.
B)Reduction takes place at the anode, which is negatively charged.
C)Reduction takes place at the cathode, which is positively charged.
D)Reduction takes place at the cathode, which is negatively charged.
E)Reduction takes place at the dynode, which is uncharged.
A)Reduction takes place at the anode, which is positively charged.
B)Reduction takes place at the anode, which is negatively charged.
C)Reduction takes place at the cathode, which is positively charged.
D)Reduction takes place at the cathode, which is negatively charged.
E)Reduction takes place at the dynode, which is uncharged.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
67
Which metal can be prepared by electrolysis of an aqueous solution of one of its salts?
A)aluminum
B)copper
C)magnesium
D)potassium
E)sodium
A)aluminum
B)copper
C)magnesium
D)potassium
E)sodium

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
68
The products of the electrolysis of molten magnesium chloride using platinum electrodes are
A)hydrogen gas and chlorine gas.
B)magnesium metal and chlorine gas.
C)magnesium metal and oxygen gas.
D)magnesium metal and hydroxide ions.
E)chlorine gas and platinum-magnesium alloy.
A)hydrogen gas and chlorine gas.
B)magnesium metal and chlorine gas.
C)magnesium metal and oxygen gas.
D)magnesium metal and hydroxide ions.
E)chlorine gas and platinum-magnesium alloy.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
69
The products of the electrolysis of aqueous magnesium chloride using platinum electrodes are
A)magnesium metal and chlorine gas.
B)magnesium metal and oxygen gas.
C)magnesium metal and hydroxide ions.
D)hydrogen gas and chlorine gas.
E)chlorine gas and platinum-magnesium alloy.
A)magnesium metal and chlorine gas.
B)magnesium metal and oxygen gas.
C)magnesium metal and hydroxide ions.
D)hydrogen gas and chlorine gas.
E)chlorine gas and platinum-magnesium alloy.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
70
When molten sodium chloride is electrolyzed, a gas is observed to form at the anode. The gas is
A)chlorine.
B)hydrogen.
C)hydrogen peroxide.
D)oxygen.
E)sodium.
A)chlorine.
B)hydrogen.
C)hydrogen peroxide.
D)oxygen.
E)sodium.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
71
When an aqueous solution of copper sulfate is electrolyzed, a gas is observed to form at the anode. The gas is
A)hydrogen.
B)hydrogen sulfide.
C)hydrogen peroxide.
D)oxygen.
E)sulfur dioxide.
A)hydrogen.
B)hydrogen sulfide.
C)hydrogen peroxide.
D)oxygen.
E)sulfur dioxide.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
72
When an aqueous solution of sodium sulfate is electrolyzed, a gas is observed to form at the anode. The gas is
A)hydrogen.
B)hydrogen sulfide.
C)hydrogen peroxide.
D)oxygen.
E)sulfur dioxide.
A)hydrogen.
B)hydrogen sulfide.
C)hydrogen peroxide.
D)oxygen.
E)sulfur dioxide.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
73
When an aqueous solution of sodium sulfate is electrolyzed, a gas is observed to form at the cathode. The gas is
A)hydrogen.
B)hydrogen sulfide.
C)hydrogen peroxide.
D)oxygen.
E)sulfur dioxide.
A)hydrogen.
B)hydrogen sulfide.
C)hydrogen peroxide.
D)oxygen.
E)sulfur dioxide.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
74
When an aqueous solution of magnesium sulfate is electrolyzed, what product is formed at the cathode?
A)hydrogen
B)hydrogen sulfide
C)magnesium
D)oxygen
E)sulfur dioxide
A)hydrogen
B)hydrogen sulfide
C)magnesium
D)oxygen
E)sulfur dioxide

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
75
When an aqueous solution of nickel sulfate is electrolyzed, what product is formed at the anode?
A)hydrogen
B)hydrogen sulfide
C)nickel
D)oxygen
E)sulfur dioxide
A)hydrogen
B)hydrogen sulfide
C)nickel
D)oxygen
E)sulfur dioxide

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
76
The half-reaction that occurs at the cathode during electrolysis of aqueous sodium iodide solution is:
A)2 H2O(l)+ 2 e- H2(g)+ 2 OH-(aq)
B)I2(aq)+ 2 e- 2 I-(aq)
C)2 I-(aq) I2(aq)+ 2 e-
D)Na+(aq)+ e- Na(s)
E)Na(s) Na+(aq)+ e-
A)2 H2O(l)+ 2 e- H2(g)+ 2 OH-(aq)
B)I2(aq)+ 2 e- 2 I-(aq)
C)2 I-(aq) I2(aq)+ 2 e-
D)Na+(aq)+ e- Na(s)
E)Na(s) Na+(aq)+ e-

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
77
The half-reaction that occurs at the cathode during electrolysis of aqueous CuCl2 solution is:
A)Cl2(g)+ 2 e- 2 Cl-(aq)
B)2 Cl(aq) Cl2(g)+ 2 e-
C)Cu2+(aq)+ 2 e- Cu(s)
D)Cu+(aq)+ e- Cu(s)
E)2 H2O + 2 e- H2(g)+ 2 OH-(aq)
A)Cl2(g)+ 2 e- 2 Cl-(aq)
B)2 Cl(aq) Cl2(g)+ 2 e-
C)Cu2+(aq)+ 2 e- Cu(s)
D)Cu+(aq)+ e- Cu(s)
E)2 H2O + 2 e- H2(g)+ 2 OH-(aq)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
78
The half-reaction that should occur at the anode during electrolysis of aqueous potassium bromide solution is:
A)Br2(g)+ 2 e- 2 Br-(aq)
B)2 Br-(aq) Br2(l)+ 2 e-
C)2 H2O O2(g)+ 4 H+(aq)+ 4 e-
D)2 H+(aq)+ e- H2(g)
E)Na+(aq)+ e- Na(s)
A)Br2(g)+ 2 e- 2 Br-(aq)
B)2 Br-(aq) Br2(l)+ 2 e-
C)2 H2O O2(g)+ 4 H+(aq)+ 4 e-
D)2 H+(aq)+ e- H2(g)
E)Na+(aq)+ e- Na(s)

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
79
The SI unit for electric current is the
A)ampere.
B)coulomb.
C)volt.
D)joule.
E)watt.
A)ampere.
B)coulomb.
C)volt.
D)joule.
E)watt.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
80
Using the same current and similar conditions, which will require the shorter length of time?
A)Depositing 0.10 mol Ag from a Ag+ solution
B)Depositing 0.10 mol Cr from a Cr3+ solution
C)Depositing 0.10 mol Cu from a Cu2+ solution
D)Depositing 0.20 mol Cu from a Cu2+ solution
E)They should all take the same time.
A)Depositing 0.10 mol Ag from a Ag+ solution
B)Depositing 0.10 mol Cr from a Cr3+ solution
C)Depositing 0.10 mol Cu from a Cu2+ solution
D)Depositing 0.20 mol Cu from a Cu2+ solution
E)They should all take the same time.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck



