Deck 7: Energy and Chemical Change
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/176
Play
Full screen (f)
Deck 7: Energy and Chemical Change
1
Which is a unit of energy, but is not the SI unit of energy?
A)joule
B)newton
C)pascal
D)watt
E)calorie
A)joule
B)newton
C)pascal
D)watt
E)calorie
calorie
2
Which is a unit of energy?
A)pascal
B)newton
C)joule
D)watt
E)ampere
A)pascal
B)newton
C)joule
D)watt
E)ampere
joule
3
Chemical energy is
A)the kinetic energy resulting from violent decomposition of energetic chemicals.
B)the heat energy associated with combustion reactions.
C)the electrical energy produced by fuel cells.
D)the potential energy that resides in chemical bonds.
E)the energy living plants receive from solar radiation.
A)the kinetic energy resulting from violent decomposition of energetic chemicals.
B)the heat energy associated with combustion reactions.
C)the electrical energy produced by fuel cells.
D)the potential energy that resides in chemical bonds.
E)the energy living plants receive from solar radiation.
the potential energy that resides in chemical bonds.
4
Calculate the kinetic energy (KE)of an object that has a mass of 5.00 × 102 g and is traveling in a straight line with a speed of 50.0 m s-1. (1 J = 1 kg m2s-2.)
A)0.625 kJ
B)1.25 kJ
C)2.5 kJ
D)6.25 kJ
E)25 kJ
A)0.625 kJ
B)1.25 kJ
C)2.5 kJ
D)6.25 kJ
E)25 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the kinetic energy (KE)of an object that has a mass of 9.00 × 102 g and is traveling in a straight line with a speed of 4.0 × 101 m s-1. (1 J = 1 kg m2s-2)
A)0.72 kJ
B)1.44 kJ
C)2.88 kJ
D)16.2 kJ
E)18 kJ
A)0.72 kJ
B)1.44 kJ
C)2.88 kJ
D)16.2 kJ
E)18 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
6
Calculate the kinetic energy (KE)of an object that has a mass of 1.200 × 103 g and is traveling in a straight line with a speed of 5.0 × 101 m s-1. (1 J = 1 kg m2s-2)
A)1.5 kJ
B)3.0 kJ
C)6.0 kJ
D)36 kJ
E)300 kJ
A)1.5 kJ
B)3.0 kJ
C)6.0 kJ
D)36 kJ
E)300 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate the kinetic energy (KE)of an object that has a mass of 2.45 kg and is traveling in a straight line with a speed of 12.0 m s-1. (1 J = 1 kg m2s-2)
A)414 J
B)353 J
C)36.0 J
D)176 J
E)465 J
A)414 J
B)353 J
C)36.0 J
D)176 J
E)465 J

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
8
How many kilojoules are equivalent to 8.18 kilocalories?
A)1.96 kJ
B)1,955 kJ
C)8,180 kJ
D)34,200 kJ
E)34.2 kJ
A)1.96 kJ
B)1,955 kJ
C)8,180 kJ
D)34,200 kJ
E)34.2 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
9
How many kilocalories are equivalent to 18.9 kilojoules?
A)79.1 kcal
B)4.52 kcal
C)9.03 kcal
D)7.91 kcal
E)34.2 kcal
A)79.1 kcal
B)4.52 kcal
C)9.03 kcal
D)7.91 kcal
E)34.2 kcal

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement is true?
A)Molecules in gases possess kinetic energy because they are in constant motion, while molecules in liquids and solids are not in constant motion and hence possess no kinetic energy.
B)Molecules in gases and liquids possess kinetic energy because they are in constant motion, while molecules in solids are not in constant motion and hence possess no kinetic energy.
C)Molecules in gases, liquids and solids possess kinetic energy because they are in constant motion.
D)Polyatomic molecules possess kinetic energy in the liquid and gaseous states because the atoms can move about in the molecule even if the molecule cannot move.
E)Because solids are rigid, their molecules do not possess kinetic energy unless the solid is melted.
A)Molecules in gases possess kinetic energy because they are in constant motion, while molecules in liquids and solids are not in constant motion and hence possess no kinetic energy.
B)Molecules in gases and liquids possess kinetic energy because they are in constant motion, while molecules in solids are not in constant motion and hence possess no kinetic energy.
C)Molecules in gases, liquids and solids possess kinetic energy because they are in constant motion.
D)Polyatomic molecules possess kinetic energy in the liquid and gaseous states because the atoms can move about in the molecule even if the molecule cannot move.
E)Because solids are rigid, their molecules do not possess kinetic energy unless the solid is melted.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
11
For a chemical reaction, where the internal energy is given the symbol E,
A)Efinal signifies the internal energy of the reactants.
B)Einitial signifies the internal energy of the products.
C) E = Eproducts Ereactants
D) E is positive if energy is released to the surroundings.
E) E is positive if energy is released by the chemical reaction.
A)Efinal signifies the internal energy of the reactants.
B)Einitial signifies the internal energy of the products.
C) E = Eproducts Ereactants
D) E is positive if energy is released to the surroundings.
E) E is positive if energy is released by the chemical reaction.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
12
Which statement is incorrect?
A)Heat can be considered the energy transferred between objects with different temperatures.
B)Internal energy is the sum of the energies of all the individual particles in a particular sample of matter.
C)If a system absorbs energy, its internal energy increases.
D)Kinetic molecular theory is related to the total molecular kinetic energy.
E)If the Kelvin temperature is doubled, the average kinetic energy is also doubled.
A)Heat can be considered the energy transferred between objects with different temperatures.
B)Internal energy is the sum of the energies of all the individual particles in a particular sample of matter.
C)If a system absorbs energy, its internal energy increases.
D)Kinetic molecular theory is related to the total molecular kinetic energy.
E)If the Kelvin temperature is doubled, the average kinetic energy is also doubled.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
13
Which statement is true?
A)A state function is one whose value for a system depends on the method of preparation of the reactants and products.
B)A state function is one whose value for a system is determined by the difference in temperature of the system, and not on the pressure of the system.
C)A state function is one whose value for the system is determined by only the pressure of the system, and not on the temperature of the system.
D)A state function is one whose value for a system is determined by the temperature of the system, and not on the composition of the system.
E)A state function is one whose value for a system is determined by the composition of the system, the volume, the temperature, and the pressure.
A)A state function is one whose value for a system depends on the method of preparation of the reactants and products.
B)A state function is one whose value for a system is determined by the difference in temperature of the system, and not on the pressure of the system.
C)A state function is one whose value for the system is determined by only the pressure of the system, and not on the temperature of the system.
D)A state function is one whose value for a system is determined by the temperature of the system, and not on the composition of the system.
E)A state function is one whose value for a system is determined by the composition of the system, the volume, the temperature, and the pressure.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
14
A freshly baked pie is placed near an open window to cool. Which of the following statements best describes this situation?
A)The pie is the system and loses heat to the surroundings.
B)The pie is the system and gains heat from the surroundings.
C)The pie is the surroundings and gains heat from the system.
D)The pie is the surroundings and loses heat to the system.
E)The pie is the surroundings and neither gains nor loses heat.
A)The pie is the system and loses heat to the surroundings.
B)The pie is the system and gains heat from the surroundings.
C)The pie is the surroundings and gains heat from the system.
D)The pie is the surroundings and loses heat to the system.
E)The pie is the surroundings and neither gains nor loses heat.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
15
A system that does not allow the transfer of mass but does allow the transfer of thermal energy would best be classified as Hint: Consider this from the thermodynamic perspective.
A)an open system.
B)a closed system.
C)an isolated system.
D)an adiabatic system.
E)an isobaric system.
A)an open system.
B)a closed system.
C)an isolated system.
D)an adiabatic system.
E)an isobaric system.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
16
A system that allows the transfer of mass and allows the transfer of thermal energy would best be classified as
A)an open system.
B)a closed system.
C)an isolated system.
D)an adiabatic system.
E)an isobaric system.
A)an open system.
B)a closed system.
C)an isolated system.
D)an adiabatic system.
E)an isobaric system.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
17
A certain oil used in industrial transformers has a density of 1.068 g mL-1 and a specific heat of 1.628 J g-1 °C-1. Calculate the heat capacity of one gallon of this oil. (1 gallon = 3.785 liters)
A)0.3747 kJ °C-1
B)0.4027 kJ °C-1
C)2.483 kJ °C-1
D)5.770 kJ °C-1
E)6.581 kJ °C?1
A)0.3747 kJ °C-1
B)0.4027 kJ °C-1
C)2.483 kJ °C-1
D)5.770 kJ °C-1
E)6.581 kJ °C?1

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
18
A certain oil used in industrial transformers has a density of 1.086 g mL-1 and a specific heat of 1.826 J g-1 °C-1. Calculate the heat capacity of one gallon of this oil. (1 gallon = 3.785 liters)
A)0.4442 kJ °C-1
B)0.5239 kJ °C-1
C)2.251 kJ °C-1
D)6.364 kJ °C-1
E)7.506 kJ °C?1
A)0.4442 kJ °C-1
B)0.5239 kJ °C-1
C)2.251 kJ °C-1
D)6.364 kJ °C-1
E)7.506 kJ °C?1

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
19
A 500.0 gram sample of aluminum is initially at 25.0 °C. It absorbs 32.60 kJ of heat from its surroundings. What is its final temperature, in °C? (specific heat = 0.9930 J g-1 °C-1 for aluminum)
A)40.4 °C
B)64.7 °C
C)65.7 °C
D)89.7 °C
E)90.7 °C
A)40.4 °C
B)64.7 °C
C)65.7 °C
D)89.7 °C
E)90.7 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
20
A 113.25 gram sample of gold is initially at 100.0 °C. It gains 20.00 J of heat from its surroundings. What is its final temperature? (specific heat of gold = 0.129 J g-1 °C-1)
A)98.6 °C
B)-98.6 °C
C)101.4 °C
D)-101.4 °C
E)96.6 °C
A)98.6 °C
B)-98.6 °C
C)101.4 °C
D)-101.4 °C
E)96.6 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
21
A 225.0 gram sample of copper absorbs 735 J of heat from its surroundings. What is the temperature change for copper sample? (specific heat = 0.387 J g-1 °C-1 for copper)
A)64.0 °C
B)8.44 °C
C)92.2 °C
D)117.3 °C
E)156.7 °C
A)64.0 °C
B)8.44 °C
C)92.2 °C
D)117.3 °C
E)156.7 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
22
A 350.0 gram sample of copper initially at 25.0 °C absorbs 12.50 kJ of heat from its surroundings. What is its final temperature? (specific heat = 0.387 J g-1 °C-1 for copper)
A)38.8 °C
B)67.2 °C
C)92.2 °C
D)117.3 °C
E)156.7 °C
A)38.8 °C
B)67.2 °C
C)92.2 °C
D)117.3 °C
E)156.7 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
23
A bomb calorimeter consists of metal parts with a heat capacity of
850.0 J °C-1 and 1.100 × 103 grams of oil with a specific heat of 2.184 J g-1 °C?1. What is the heat capacity, in joules per degree, of the entire calorimeter?
A)1354 J °C-1
B)1952 J °C-1
C)2956 J °C-1
D)3252 J °C-1
E)4259 J °C-1
850.0 J °C-1 and 1.100 × 103 grams of oil with a specific heat of 2.184 J g-1 °C?1. What is the heat capacity, in joules per degree, of the entire calorimeter?
A)1354 J °C-1
B)1952 J °C-1
C)2956 J °C-1
D)3252 J °C-1
E)4259 J °C-1

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
24
A bomb calorimeter consists of metal parts with a heat capacity of 925.0 J °C-1 and 1.100 × 103 grams of oil with a specific heat of 2.814 J g-1 °C-1. What is the heat capacity, in joules per degree, of the entire calorimeter?Hint: Determine the heat capacity of the oil and the metal separately first.
A)1321 J °C-1
B)2028 J °C-1
C)3703 J °C-1
D)4020 J °C-1
E)5698 J °C-1
A)1321 J °C-1
B)2028 J °C-1
C)3703 J °C-1
D)4020 J °C-1
E)5698 J °C-1

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
25
A bomb calorimeter consists of metal parts with a heat capacity of 950.0 J °C-1 and 8.50 × 102 grams of oil with a specific heat of 2.418 J g-1 °C-1. Calculate the amount of heat energy required, in kJ, to raise the temperature of the calorimeter from 25.00 °C to 31.60 °C.Hint: Determine the amount of energy required to raise the temperature of oil and metal separately first.
A)4.91 kJ
B)11.9 kJ
C)19.8 kJ
D)20.8 kJ
E)28.7 kJ
A)4.91 kJ
B)11.9 kJ
C)19.8 kJ
D)20.8 kJ
E)28.7 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
26
A bomb calorimeter consists of metal parts with a heat capacity of 925.0 J °C-1 and 1.100 × 103 grams of oil with a specific heat of 2.184 J g-1 °C-1. Calculate the heat required, in kJ, to raise the temperature of the calorimeter from 24.40 °C to 29.75 °C.Hint: Determine the amount of energy required to raise the temperature of oil and metal separately first.
A)0.827 kJ
B)7.64 kJ
C)17.8 kJ
D)23.7 kJ
E)99.0 kJ
A)0.827 kJ
B)7.64 kJ
C)17.8 kJ
D)23.7 kJ
E)99.0 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
27
A 113.25 gram sample of gold is initially at 100.0 °C. It loses 20.00 J of heat to its surroundings. What is its final temperature? (specific heat of gold = 0.129 J g-1 °C-1)
A)98.6 °C
B)-98.6 °C
C)94.6 °C
D)-94.6 °C
E)96.6 °C
A)98.6 °C
B)-98.6 °C
C)94.6 °C
D)-94.6 °C
E)96.6 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
28
A 25.00 gram gold ingot and a 30.00 gram block of copper are placed in 100.00 grams of water. If the initial temperatures of the gold, copper, and water were 95.0 °C, 85.0 °C, and25.0 °C, respectively, what would the final temperature of the entire system be? The specific heats of gold, copper, and liquid water are 0.129, 0.387, and 4.18 J g-1 °C-1, respectively.Hint: Set up your equation for each metal and water individually before combining to determine the final temperature of the entire system.
A)26.0 °C
B)28.2 °C
C)23.1 °C
D)-27.1 °C
E)27.1 °C
A)26.0 °C
B)28.2 °C
C)23.1 °C
D)-27.1 °C
E)27.1 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
29
A 25.00 gram pellet of lead (specific heat = 0.128 J g-1 °C-1)at 25 °C is added to 95.3 g of boiling water (specific heat of 4.18 J g-1 °C-1)at 100 °C in an insulated cup. What is the expected final temperature of the water?
A)26.6 °C
B)62.5 °C
C)84.4 °C
D)99.4 °C
E)100.6 °C
A)26.6 °C
B)62.5 °C
C)84.4 °C
D)99.4 °C
E)100.6 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
30
A 55.00 gram pellet of lead at 25 °C is added to 58.5 g of boiling water (specific heat of 4.18 J g-1 °C-1)at 100 °C in an insulated cup. If the final temperature of the water in the cup is97.9 °C, what is the specific heat of lead?
A)17.8 J g-1 °C-1
B)0.128 J g-1 °C-1
C)4.17 J g-1 °C-1
D)22.2 J g-1 °C-1
E)0.372 J g-1 °C-1
A)17.8 J g-1 °C-1
B)0.128 J g-1 °C-1
C)4.17 J g-1 °C-1
D)22.2 J g-1 °C-1
E)0.372 J g-1 °C-1

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
31
A sample of chromium weighing 254 g was initially at a temperature of 25.88 °C. It required 843 joules of heat energy to increase the temperature to 32.75 °C. What is the molar heat capacity of the chromium? Hint: It might help to calculate the specific heat capacity first.
A)21.6 J mol-1 °C-1
B)25.1 J mol-1 °C-1
C)33.2 J mol-1 °C-1
D)37.3 J mol-1 °C-1
E)17.4 J mol-1 °C-1
A)21.6 J mol-1 °C-1
B)25.1 J mol-1 °C-1
C)33.2 J mol-1 °C-1
D)37.3 J mol-1 °C-1
E)17.4 J mol-1 °C-1

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
32
A coffee cup calorimeter contains 480.0 grams of water at 25.00 °C. To it are added: 380.0 grams of water at 53.5 °C
525.0 grams of water at 65.5 °C Assuming the heat absorbed by the coffee cup is negligible, calculate the expected final temperature of the water. The specific heat of water is 4.184 J g-1 °C-1.Hint: Take this problem one step (one water addition)at a time.
A)38.2 °C
B)48.2 °C
C)67.6 °C
D)88.7 °C
E)94.4 °C
525.0 grams of water at 65.5 °C Assuming the heat absorbed by the coffee cup is negligible, calculate the expected final temperature of the water. The specific heat of water is 4.184 J g-1 °C-1.Hint: Take this problem one step (one water addition)at a time.
A)38.2 °C
B)48.2 °C
C)67.6 °C
D)88.7 °C
E)94.4 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
33
A coffee cup calorimeter contains 525.0 grams of water at 25.0 °C. To it are added: 350.0 grams of water at 48.3 °C
480.0 grams of water at 63.8 °C Neglecting the heat absorbed by the coffee cup, calculate the final temperature of the water. The specific heat of water is 4.184 J g-1 °C-1.Hint: Take this problem one step (one water addition)at a time.
A)39.6 °C
B)45.7 °C
C)44.8 °C
D)66.7 °C
E)92.4 °C
480.0 grams of water at 63.8 °C Neglecting the heat absorbed by the coffee cup, calculate the final temperature of the water. The specific heat of water is 4.184 J g-1 °C-1.Hint: Take this problem one step (one water addition)at a time.
A)39.6 °C
B)45.7 °C
C)44.8 °C
D)66.7 °C
E)92.4 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
34
A constant pressure calorimeter consists of metal parts with a heat capacity of 850.0 J °C-1 and 1.050 × 103 grams of oil with a specific heat of 2.148 J g-1 °C-1. Both are at 24.50 °C. A 5.00 × 102 g copper slug, at 220.0 °C is added. What is the final temperature? Specific heat of Cu = 0.3874 J g-1 °C-1.Hint: Take this problem one step at a time. Look at the energy required to raise the temperature of oil and metal separately first before considering the entire system.
A)33.4 °C
B)36.0 °C
C)36.8 °C
D)89.7 °C
E)120.5 °C
A)33.4 °C
B)36.0 °C
C)36.8 °C
D)89.7 °C
E)120.5 °C

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
35
A constant pressure calorimeter has metal parts (heat capacity of 850.0 J °C-1)and 1.100 × 103 grams of oil (specific heat = 2.184 J g-1 °C-1), both at 24.50°C. Adding a 4.60 × 102 g slug, at 240.0°C, caused the temperature to rise to 32.5 °C. Find the specific heat of the metal.Hint: Consider the amount of energy that was required to raise the temperature of the entire system.
A)0.236 J g-1 °C-1
B)0.273 J g-1 °C-1
C)0.309 J g-1 °C-1
D)0.357 J g-1 °C-1
E)2.28 J g-1 °C-1
A)0.236 J g-1 °C-1
B)0.273 J g-1 °C-1
C)0.309 J g-1 °C-1
D)0.357 J g-1 °C-1
E)2.28 J g-1 °C-1

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
36
A constant pressure calorimeter has metal parts (heat capacity of 925.0 J °C-1)and 1.100 × 103 grams of oil (specific heat = 2.824 J g-1 °C-1), both at 25.40°C. Adding a 5.50 × 102 g slug at 220.0°C, caused the temperature to rise to 35.2 °C. Find the specific heat of the metal.Hint: Consider the amount of energy that was required to raise the temperature of the entire system.
A)0.365 J g-1 °C-1
B)0.389 J g-1 °C-1
C)0.395 J g-1 °C-1
D)0.551 J g-1 °C-1
E)1.20 J g-1 °C-1
A)0.365 J g-1 °C-1
B)0.389 J g-1 °C-1
C)0.395 J g-1 °C-1
D)0.551 J g-1 °C-1
E)1.20 J g-1 °C-1

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
37
During an exothermic chemical reaction,
A)a system becomes warmer and the chemical substances undergo an increase in potential energy.
B)a system becomes warmer and the chemical substances undergo a decrease in potential energy.
C)a system becomes cooler and the chemical substances undergo an increase in potential energy.
D)a system becomes cooler and the chemical substances undergo a decrease in potential energy.
E)a system becomes warmer and additional heat is gained from the surroundings.
A)a system becomes warmer and the chemical substances undergo an increase in potential energy.
B)a system becomes warmer and the chemical substances undergo a decrease in potential energy.
C)a system becomes cooler and the chemical substances undergo an increase in potential energy.
D)a system becomes cooler and the chemical substances undergo a decrease in potential energy.
E)a system becomes warmer and additional heat is gained from the surroundings.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
38
During an endothermic chemical reaction,
A)a system becomes warmer and the chemical substances undergo an increase in potential energy.
B)a system becomes warmer and the chemical substances undergo a decrease in potential energy.
C)a system becomes cooler and the chemical substances undergo an increase in potential energy.
D)a system becomes cooler and the chemical substances undergo a decrease in potential energy.
E)a system becomes warmer and additional heat is gained from the surroundings.
A)a system becomes warmer and the chemical substances undergo an increase in potential energy.
B)a system becomes warmer and the chemical substances undergo a decrease in potential energy.
C)a system becomes cooler and the chemical substances undergo an increase in potential energy.
D)a system becomes cooler and the chemical substances undergo a decrease in potential energy.
E)a system becomes warmer and additional heat is gained from the surroundings.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
39
Which statement is generally true?
A)A chemical reaction involves only the making of chemical bonds.
B)A chemical reaction involves only the breaking of chemical bonds.
C)Breaking weak chemical bonds require a relatively large amount of energy.
D)When bonds break in chemical reactions, the potential energy of the system tends to increase.
E)When bonds break in chemical reactions, the potential energy of the system tends to decrease.
A)A chemical reaction involves only the making of chemical bonds.
B)A chemical reaction involves only the breaking of chemical bonds.
C)Breaking weak chemical bonds require a relatively large amount of energy.
D)When bonds break in chemical reactions, the potential energy of the system tends to increase.
E)When bonds break in chemical reactions, the potential energy of the system tends to decrease.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
40
For a change in a system that takes place at constant pressure, which statement below is true?
A) H = E
B) H = qp P V
C) H = E qp
D) H = qp
E) E = qp
A) H = E
B) H = qp P V
C) H = E qp
D) H = qp
E) E = qp

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
41
For a chemical reaction taking place at constant pressure, which one of the following is true?
A) Hsystem = (Kinetic Energy)system + (Potential Energy)system
B) Hsystem = (Kinetic Energy)system - (Potential Energy)system
C) Hsystem = Esystem qp
D) Hsystem = Esystem + P Vsystem
E) Hsystem = Esystem + qp
A) Hsystem = (Kinetic Energy)system + (Potential Energy)system
B) Hsystem = (Kinetic Energy)system - (Potential Energy)system
C) Hsystem = Esystem qp
D) Hsystem = Esystem + P Vsystem
E) Hsystem = Esystem + qp

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
42
An endothermic reaction is one in that there is
A)a positive value for the work done by the system (w > 0 joules).
B)a negative value for the work done by the system (w < 0 joules).
C)a negative value for H ( H < 0 joules).
D)a positive value for H ( H > 0 joules).
E)a negative value for E ( E > 0 joules).
A)a positive value for the work done by the system (w > 0 joules).
B)a negative value for the work done by the system (w < 0 joules).
C)a negative value for H ( H < 0 joules).
D)a positive value for H ( H > 0 joules).
E)a negative value for E ( E > 0 joules).

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
43
In the course of measuring fuel content values, a reaction for the conversion of crude oil fuel into water and carbon dioxide is carried out in two steps Crude fuel oil + oxygen CO(g)+ H2O
CO(g)+ oxygen CO2(g)The net reaction taking place is: crude fuel oil + oxygen CO2(g)+ H2O. A large fraction of the raw material is converted in one step, while the second step is to collect the fraction that was just partially burned the first time. For the overall or net process, which statement below is always true?
A) H is independent of the time interval between the two steps, but dependent on the fraction that had to be converted in two steps.
B) H is dependent on the time interval between the two steps, but dependent on the fraction that had to be converted in two steps.
C) H is independent of the time interval between the two steps and also independent of the fraction that had to be converted in two steps.
D) H is dependent on the time interval between the two steps, but independent of the fraction that had to be converted in two steps.
E) H is independent of the time interval between the two steps, but dependent on the time required for completion of the entire process.
CO(g)+ oxygen CO2(g)The net reaction taking place is: crude fuel oil + oxygen CO2(g)+ H2O. A large fraction of the raw material is converted in one step, while the second step is to collect the fraction that was just partially burned the first time. For the overall or net process, which statement below is always true?
A) H is independent of the time interval between the two steps, but dependent on the fraction that had to be converted in two steps.
B) H is dependent on the time interval between the two steps, but dependent on the fraction that had to be converted in two steps.
C) H is independent of the time interval between the two steps and also independent of the fraction that had to be converted in two steps.
D) H is dependent on the time interval between the two steps, but independent of the fraction that had to be converted in two steps.
E) H is independent of the time interval between the two steps, but dependent on the time required for completion of the entire process.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
44
A chemical reaction took place in a 6 liter cylindrical enclosure fitted with a piston (like the cylinder in an internal combustion engine). Over the time required for the reaction to be completed, the volume of the system changed from 0.400 liters to 3.20 liters. Which of the following statements below is true?
A)Work was performed on the system.
B)Work was performed by the system.
C)The internal energy of the system increased.
D)The internal energy of the system decreased.
E)The internal energy of the system remained unchanged.
A)Work was performed on the system.
B)Work was performed by the system.
C)The internal energy of the system increased.
D)The internal energy of the system decreased.
E)The internal energy of the system remained unchanged.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
45
A chemical reaction took place in a 5 liter cylindrical enclosure fitted with a piston (like the cylinder in an internal combustion engine). Over the time required for the reaction to be completed, the volume of the system changed from 1.40 liters to 3.70 liters. Which of the following statements below is true?
A)The enthalpy of the system remained unchanged.
B)The enthalpy of the system decreased.
C)The enthalpy of the system increased.
D)Work was performed by the system.
E)Work was performed on the system.
A)The enthalpy of the system remained unchanged.
B)The enthalpy of the system decreased.
C)The enthalpy of the system increased.
D)Work was performed by the system.
E)Work was performed on the system.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
46
A closed, uninsulated system was fitted with a movable piston. The introduction of 430 J of heat caused the system to expand, doing 238 J of work in the process against a constant pressure of 101 kPa (kilopascals). What is the value of E for this process?
A)(430 + 238)joules
B)(430 - 238)joules
C)(238 - 430)joules
D)430 joules
E)(-238 - 430)joules
A)(430 + 238)joules
B)(430 - 238)joules
C)(238 - 430)joules
D)430 joules
E)(-238 - 430)joules

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
47
A closed, uninsulated system was fitted with a movable piston. Introduction of 430 J of heat caused the system to expand, doing 238 J of work in the process against a constant pressure of 101 kPa (kilopascals). What is the value of H for this process?
A)(430 + 238)joules
B)(430- 238)joules
C)(238 - 430)joules
D)430 joules
E)(-238 - 430)joules
A)(430 + 238)joules
B)(430- 238)joules
C)(238 - 430)joules
D)430 joules
E)(-238 - 430)joules

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
48
A closed, uninsulated system was fitted with a movable piston. Introduction of 483 J of heat caused the system to expand, doing 320 J of work in the process against a constant pressure of 101 kPa (kilopascals). What is the value of E for this process?
A)(483 + 320)joules
B)(483 - 320)joules
C)(320 - 483)joules
D)483 joules
E)(-320 - 483)joules
A)(483 + 320)joules
B)(483 - 320)joules
C)(320 - 483)joules
D)483 joules
E)(-320 - 483)joules

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
49
A closed, uninsulated system was fitted with a movable piston. Introduction of 483 J of heat caused the system to expand, doing 320 J of work in the process against a constant pressure of 101 kPa (kilopascals). What is the value of H for this process?
A)(483 + 320)joules
B)(483 - 320)joules
C)(320 - 483)joules
D)483 joules
E)(-320 - 483)joules
A)(483 + 320)joules
B)(483 - 320)joules
C)(320 - 483)joules
D)483 joules
E)(-320 - 483)joules

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
50
For the reaction, D2(s)+ 2 AX(g) A2(g)+ 2 DX(g)taking place in an insulated system, the enthalpy of the reactants is lower than that of the products. Which one of the following is true for the system?
A)The energy of the system decreases as the reactants are converted to products.
B)The energy of the system increases as the reactants are converted to products.
C)The total energy of the system decreases as the reactants are converted to products.
D)The total mass of the system decreases as the reactants are converted to products.
E)The total mass of the system increases as the reactants are converted to products.
A)The energy of the system decreases as the reactants are converted to products.
B)The energy of the system increases as the reactants are converted to products.
C)The total energy of the system decreases as the reactants are converted to products.
D)The total mass of the system decreases as the reactants are converted to products.
E)The total mass of the system increases as the reactants are converted to products.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
51
When pure sodium hydroxide is dissolved in water, heat is evolved. In a laboratory experiment to measure the molar heat of solution of sodium hydroxide, the following procedure was followed. To a calorimeter containing 3.00 × 102 g of water at 20.00 °C, 10.65 g of NaOH, also at 20.00 °C was added. The temperature of the solution, which was monitored by a digital thermometer with negligible heat capacity, increased to 28.50 °C. If the specific heat of the mixture is 4.184 J g-1 °C-1, and the small heat capacity of the calorimeter is ignored, what is the heat evolved, per mole of sodium hydroxide?Hint: Do not ignore the specific heat of water given in this problem and be sure to convert NaOH to moles before proceeding.
A)-37.4 kJ
B)-41.5 kJ
C)-45.5 kJ
D)-90.5 kJ
E)-153 kJ
A)-37.4 kJ
B)-41.5 kJ
C)-45.5 kJ
D)-90.5 kJ
E)-153 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
52
When pure sulfuric acid is dissolved in water, heat is evolved. In a laboratory experiment to measure the molar heat of solution of sulfuric acid, the following procedure was followed. To a calorimeter containing 3.00 × 102 g of water at 20.00 °C, 10.65 g of H2SO4, also at 20.00 °C was added. The temperature of the solution, which was monitored by a digital thermometer with negligible heat capacity, increased to 26.55 °C. If the specific heat of the mixture is4.184 J g-1 °C-1, and the small heat capacity of the calorimeter is ignored, what is the heat evolved, per mole of sulfuric acid?Hint: Do not ignore the specific heat of water given in this problem and be sure to convert H2SO4 to moles before proceeding.
A)-27.4 kJ
B)-72.8 kJ
C)-78.4 kJ
D)-84.6 kJ
E)-292 kJ
A)-27.4 kJ
B)-72.8 kJ
C)-78.4 kJ
D)-84.6 kJ
E)-292 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
53
When 0.250 moles of LiCl are added to 200.0 g of water in a constant pressure calorimeter a temperature change of +11.08 °C is observed. Given that the specific heat of the resulting solution is 4.184 J g-1 °C and we can ignore the small amount of energy absorbed by the calorimeter, what is the molar enthalpy of solution ( Hsol)for LiCl?Hint: Do not ignore the specific heat of water in this problem.
A)37.1 kJ/mol
B)-185.4 kJ/mol
C)-37.1 kJ/mol
D)18.5 kJ/mol
E)-18.5 kJ/mol
A)37.1 kJ/mol
B)-185.4 kJ/mol
C)-37.1 kJ/mol
D)18.5 kJ/mol
E)-18.5 kJ/mol

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
54
What would be the "standard state"for acetic acid in solution?
A)A solution with a concentration of 1.000 M.
B)A solution at 1.000 bar of pressure.
C)A solution at 1.000 Pascal of pressure.
D)A solution at 298 K.
E)A solution that is in the solid state.
A)A solution with a concentration of 1.000 M.
B)A solution at 1.000 bar of pressure.
C)A solution at 1.000 Pascal of pressure.
D)A solution at 298 K.
E)A solution that is in the solid state.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
55
What would be the "standard state"for hydrogen gas at room temperature?
A)A gas sample with a concentration of 1.000 M.
B)A gas sample at 1.000 atm of pressure.
C)A gas sample at 1.000 Pascal of pressure.
D)A liquid solution at 298 K.
E)A liquid solution at 1.000 atm.
A)A gas sample with a concentration of 1.000 M.
B)A gas sample at 1.000 atm of pressure.
C)A gas sample at 1.000 Pascal of pressure.
D)A liquid solution at 298 K.
E)A liquid solution at 1.000 atm.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
56
When nitrogen gas reacts with hydrogen gas to form ammonia, 92.38 kJ of heat are given off for each mole of nitrogen gas consumed, under constant pressure and standard conditions. What is the correct value for the standard enthalpy of reaction in the thermochemical equation below when 0.750 mol of hydrogen reacts? N2(g)+ 3H2(s) 2 NH3(g)
A)+34.5 kJ
B)-98.3 kJ
C)+59.2 kJ
D)-59.2 kJ
E)-23.1 kJ
A)+34.5 kJ
B)-98.3 kJ
C)+59.2 kJ
D)-59.2 kJ
E)-23.1 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
57
When aluminum metal reacts with iron(III)oxide to form aluminum oxide and iron metal, 429.6 kJ of heat are given off for each mole of aluminum metal consumed, under constant pressure and standard conditions. What is the correct value for the standard enthalpy of reaction in the thermochemical equation below?
2 Al(s)+ Fe2O3(s) 2 Fe(s)+ Al2O3(s)
A)+429.6 kJ
B)-429.6 kJ
C)+859.2 kJ
D)-859.2 kJ
E)-1289 kJ
2 Al(s)+ Fe2O3(s) 2 Fe(s)+ Al2O3(s)
A)+429.6 kJ
B)-429.6 kJ
C)+859.2 kJ
D)-859.2 kJ
E)-1289 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
58
The thermochemical equation for the reaction between dinitrogen monoxide and oxygen to produce nitrogen dioxide is shown below. Write the thermochemical equation for the reaction when 1.00 mole of nitrogen dioxide is formed. 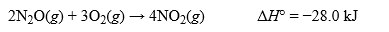
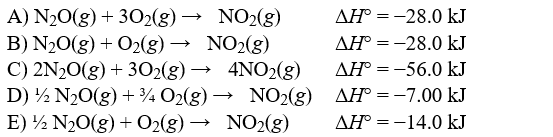
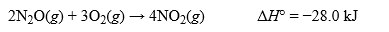
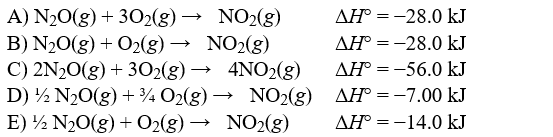

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
59
The combustion of butane, C4H10, is given as: 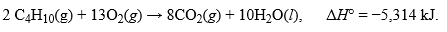 .How many grams of butane must be reacted by this reaction to release 15,285 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.
.How many grams of butane must be reacted by this reaction to release 15,285 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.
A)167.2 g
B)83.62 g
C)668.8 g
D)333.7g
E)33.09 g
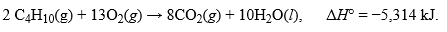 .How many grams of butane must be reacted by this reaction to release 15,285 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.
.How many grams of butane must be reacted by this reaction to release 15,285 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.A)167.2 g
B)83.62 g
C)668.8 g
D)333.7g
E)33.09 g

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
60
Propane is often used to heat homes. The combustion of propane follows the following reaction: 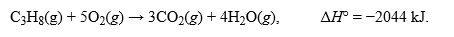 .How many grams of propane must be reacted by this reaction to release 7563 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.
.How many grams of propane must be reacted by this reaction to release 7563 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.
A)3.70 g
B)44.1 g
C)81.6 g
D)243.4 g
E)162.8 g
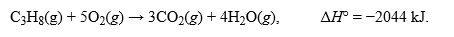 .How many grams of propane must be reacted by this reaction to release 7563 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.
.How many grams of propane must be reacted by this reaction to release 7563 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.A)3.70 g
B)44.1 g
C)81.6 g
D)243.4 g
E)162.8 g

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
61
For the reaction below: 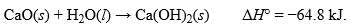 .How many grams of CaO must be reacted by this reaction to release 1050 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start
.How many grams of CaO must be reacted by this reaction to release 1050 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start
A)16.2 g
B)907 g
C)1817 g
D)454 g
E)56.1 g
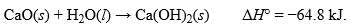 .How many grams of CaO must be reacted by this reaction to release 1050 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start
.How many grams of CaO must be reacted by this reaction to release 1050 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to startA)16.2 g
B)907 g
C)1817 g
D)454 g
E)56.1 g

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
62
When nitrogen gas reacts with hydrogen gas to form ammonia, 92.38 kJ of heat is given off for each mole of nitrogen gas consumed, under constant pressure and standard conditions. What is the value of H° for the reverse of the reaction shown? 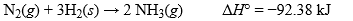
A)+34.5 kJ
B)-46.19 kJ
C)+59.2 kJ
D)-59.2 kJ
E)+92.38 kJ
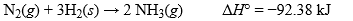
A)+34.5 kJ
B)-46.19 kJ
C)+59.2 kJ
D)-59.2 kJ
E)+92.38 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the following thermochemical equation: 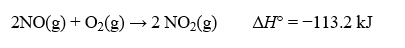 Calculate H° for the reaction below:
Calculate H° for the reaction below: 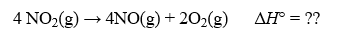
A)+334.5 kJ
B)-146.19 kJ
C)+226.4 kJ
D)-509.2 kJ
E)+192.38 kJ
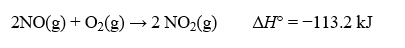 Calculate H° for the reaction below:
Calculate H° for the reaction below: 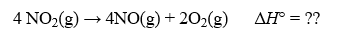
A)+334.5 kJ
B)-146.19 kJ
C)+226.4 kJ
D)-509.2 kJ
E)+192.38 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
64
Determine the enthalpy change, ?H, for the reaction, N2(g)+ 2H2(g) N2H4(l), given the following thermochemical equations: 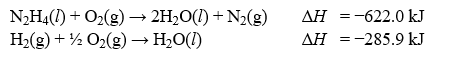
A)-151.7 kJ
B)-236.2 kJ
C)+106.1 kJ
D)+50.2 kJ
E)+567.4 kJ
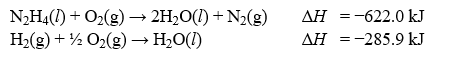
A)-151.7 kJ
B)-236.2 kJ
C)+106.1 kJ
D)+50.2 kJ
E)+567.4 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
65
Determine the enthalpy change, H, for the reaction, W(s)+ C(s)? WC(s), given the following thermochemical equations: 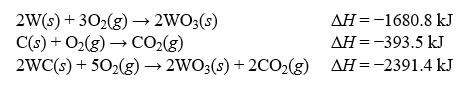
A)+33.3 kJ
B)-38.2 kJ
C)+106.1 kJ
D)-52.9 kJ
E)+177.4 kJ
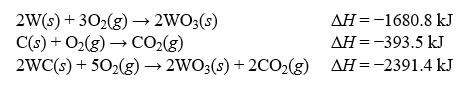
A)+33.3 kJ
B)-38.2 kJ
C)+106.1 kJ
D)-52.9 kJ
E)+177.4 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
66
Determine the enthalpy change, ?H, for the reaction, CS2(l)+ 3O2(g) CO2(g)+ 2SO2(g), given the following thermochemical equations: 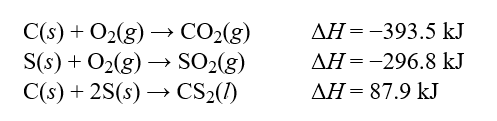 Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
A)+778.2 kJ
B)-602.4 kJ
C)-1075 kJ
D)-778.2 kJ
E)+602.4 kJ
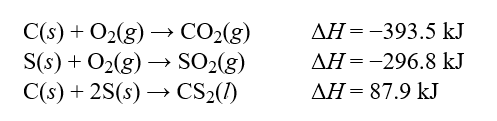 Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.A)+778.2 kJ
B)-602.4 kJ
C)-1075 kJ
D)-778.2 kJ
E)+602.4 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
67
Determine the standard enthalpy change, H°, for the reaction, CCl4(l)+ 4HCl(g) CH4(g)+ 4Cl2(g), given the following thermochemical equations: 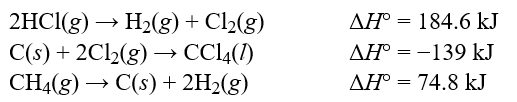 Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
A)+55.3 kJ
B)-187 kJ
C)+101 kJ
D)-179 kJ
E)+433 kJ
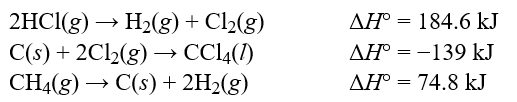 Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.A)+55.3 kJ
B)-187 kJ
C)+101 kJ
D)-179 kJ
E)+433 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
68
The thermochemical equation that is associated with H°f, the standard enthalpy of formation for HCl(g), is
A)H(g)+ Cl(g) HCl(g).
B)H2(g)+ Cl2(g) 2 HCl(g).
C)½ H2(g)+ ½ Cl2(g) HCl(g).
D)H2(g)+ Cl2(l) 2 HCl(g).
E)½ H2(g)+ ½ Cl2(l) HCl(g).
A)H(g)+ Cl(g) HCl(g).
B)H2(g)+ Cl2(g) 2 HCl(g).
C)½ H2(g)+ ½ Cl2(g) HCl(g).
D)H2(g)+ Cl2(l) 2 HCl(g).
E)½ H2(g)+ ½ Cl2(l) HCl(g).

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
69
The thermochemical equation that is associated with H°f, the standard enthalpy of formation for H2O(g), is
A)2 H(g)+ O(g) H2O(g).
B)H2O2(l)+ ½O(g) H2O(g).
C)2 H2(g)+ O2(g) 2 H2O(g).
D)H2(g)+ ½ O2(g) H2O(g).
E)2 H(g)+ ½ O2(g) H2O(g).
A)2 H(g)+ O(g) H2O(g).
B)H2O2(l)+ ½O(g) H2O(g).
C)2 H2(g)+ O2(g) 2 H2O(g).
D)H2(g)+ ½ O2(g) H2O(g).
E)2 H(g)+ ½ O2(g) H2O(g).

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
70
The thermochemical equation that is associated with H°f, the standard enthalpy of formation for glucose, C6H12O6(s), is
A)6 C(s)+ 6 H2O(l) C6H12O6(s).
B)6 C(s)+ 12 H(g)+ 6 O(g) C6H12O6(s).
C)6 C(s)+ 6 H2(g)+ 3 O2(g) C6H12O6(s).
D)2 C2H5OH(l)+ 2 CO2(g) C6H12O6(s).
E)6 C(g)+ 6 H2(g)+ 3 O2(g) C6H12O6(s).
A)6 C(s)+ 6 H2O(l) C6H12O6(s).
B)6 C(s)+ 12 H(g)+ 6 O(g) C6H12O6(s).
C)6 C(s)+ 6 H2(g)+ 3 O2(g) C6H12O6(s).
D)2 C2H5OH(l)+ 2 CO2(g) C6H12O6(s).
E)6 C(g)+ 6 H2(g)+ 3 O2(g) C6H12O6(s).

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
71
The thermochemical equation that is associated with H°f, the standard enthalpy of formation for acetic acid, C2H4O2(l), is
A)C2(s)+ 4 H(g)+ O2(g) C2H4O2(l).
B)2 C(g)+ 4 H(g)+ 2 O(g) C2H4O2(l).
C)C2(s)+ 2 H2(g)+ O2(g) C2H4O2(l).
D)2 C(s)+ 2 H2(g)+ O2(g) C2H4O2(l).
E)C(s)+ H2(g)+ O2(g) C2H4O2(l).
A)C2(s)+ 4 H(g)+ O2(g) C2H4O2(l).
B)2 C(g)+ 4 H(g)+ 2 O(g) C2H4O2(l).
C)C2(s)+ 2 H2(g)+ O2(g) C2H4O2(l).
D)2 C(s)+ 2 H2(g)+ O2(g) C2H4O2(l).
E)C(s)+ H2(g)+ O2(g) C2H4O2(l).

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
72
The thermochemical equation that is associated with H°f, the standard enthalpy of formation for urea, CO(NH2)2(s), is
A)CO(g)+ 2 NH3(g) CO(NH2)2(s)+ H2(g).
B)CO(g)+ 2 H2(g)+ N2(g) CO(NH2)2(s).
C)C(s)+ O(g)+ N2(g)+ 2 H2(g) CO(NH2)2(s).
D)C(s)+ ½ O2(g)+ N2(g)+ 2 H2(g) CO(NH2) 2(s).
E)C(s)+ ½ O2(g)+ 2 NH2(g) CO(NH2) 2(s).
A)CO(g)+ 2 NH3(g) CO(NH2)2(s)+ H2(g).
B)CO(g)+ 2 H2(g)+ N2(g) CO(NH2)2(s).
C)C(s)+ O(g)+ N2(g)+ 2 H2(g) CO(NH2)2(s).
D)C(s)+ ½ O2(g)+ N2(g)+ 2 H2(g) CO(NH2) 2(s).
E)C(s)+ ½ O2(g)+ 2 NH2(g) CO(NH2) 2(s).

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
73
Given the equation for a hypothetical reaction, 3A + 4B 4C + 7D, and the following standard enthalpies of formation, 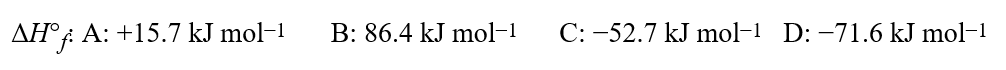 calculate the standard enthalpy of reaction, in kJ, for the reaction shown.
calculate the standard enthalpy of reaction, in kJ, for the reaction shown.
A)-53.6 kJ
B)-413.5 kJ
C)-515.6 kJ
D)-853.6 kJ
E)-908.4 kJ
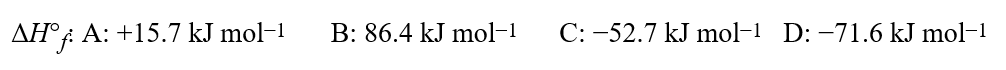 calculate the standard enthalpy of reaction, in kJ, for the reaction shown.
calculate the standard enthalpy of reaction, in kJ, for the reaction shown.A)-53.6 kJ
B)-413.5 kJ
C)-515.6 kJ
D)-853.6 kJ
E)-908.4 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
74
Given the equation for a hypothetical reaction, 5A + 3B 7C + 3D, and the following standard enthalpies of formation, 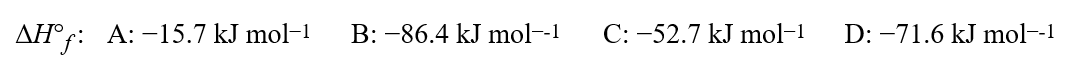 what is the standard enthalpy of reaction, in kJ for the reaction shown?
what is the standard enthalpy of reaction, in kJ for the reaction shown?
A)+26.6 kJ
B)-53.6 kJ
C)-198.8 kJ
D)-246.0 kJ
E)-413.5 kJ
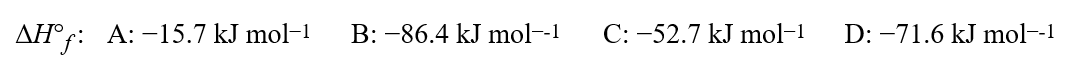 what is the standard enthalpy of reaction, in kJ for the reaction shown?
what is the standard enthalpy of reaction, in kJ for the reaction shown?A)+26.6 kJ
B)-53.6 kJ
C)-198.8 kJ
D)-246.0 kJ
E)-413.5 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
75
Given the equation for the reaction, CO2(g)+ 4H2(g) CH4(g)+ 2H2O(g), and the following standard enthalpies of formation, H°f : CO2(g): -393.5 kJ mol-1
CH4(g): ?74.8 kJ mol-1
H2O(g): -241.8 kJ mol-1
H2O(l): 285.8 kJ mol-1 what is the standard enthalpy of reaction, in kJ for the reaction shown?
A)-164.9 kJ
B)+76.9 kJ
C)-164.5 kJ
D)+978.3 kJ
E)+995.9 kJ
CH4(g): ?74.8 kJ mol-1
H2O(g): -241.8 kJ mol-1
H2O(l): 285.8 kJ mol-1 what is the standard enthalpy of reaction, in kJ for the reaction shown?
A)-164.9 kJ
B)+76.9 kJ
C)-164.5 kJ
D)+978.3 kJ
E)+995.9 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
76
Ammonia gas reacts with molecular oxygen to produce nitrogen dioxide gas and water vapor. Given the following standard enthalpies of formation, H°f:NH3 (g): -80.3 kJ mol-1
NO2 (g): +33.2 kJ mol-1
H2O(g): -241.8 kJ mol-1
H2O(l): -285.8 kJ mol-1 What is the standard enthalpy of reaction, in kJ for the reaction shown?Hint: Write the balanced reaction and be sure to pay attention to your coefficients when calculating the standard enthalpy of the overall reaction.
A)-172.3 kJ
B)-128.3 kJ
C)+157.5 kJ
D)-996.8 kJ
E)+1003.8 kJ
NO2 (g): +33.2 kJ mol-1
H2O(g): -241.8 kJ mol-1
H2O(l): -285.8 kJ mol-1 What is the standard enthalpy of reaction, in kJ for the reaction shown?Hint: Write the balanced reaction and be sure to pay attention to your coefficients when calculating the standard enthalpy of the overall reaction.
A)-172.3 kJ
B)-128.3 kJ
C)+157.5 kJ
D)-996.8 kJ
E)+1003.8 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
77
The standard enthalpy of combustion for xylene, C8H10(l), is -3908 kJ mol-1. Using this information and the standard enthalpies of formation of the following, H°f:H2O(l)= -285.9 kJ mol-1; CO2(g)= -393.5 kJ mol-1, calculate the standard enthalpy of formation of C8H10(l), in kJ mol-1.Hint: To begin, determine the thermochemical equation associated with the standard enthalpy for the formation of xylene.
A)-669.5 kJ
B)+3228.6 kJ
C)-3228.6 kJ
D)+4587.4 kJ
E)+8485.5 kJ
A)-669.5 kJ
B)+3228.6 kJ
C)-3228.6 kJ
D)+4587.4 kJ
E)+8485.5 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
78
A chemical compound has a molecular weight of 89.05 g/mole. 1.400 grams of this compound underwent complete combustion under constant pressure conditions in a calorimeter with a heat capacity of 2.980 × 103 J °C-1. The temperature went up by 11.95 degrees. Calculate the standard heat of combustion of the compound.Hint: Remember to use moles as an intermediary when determining the standard heat of combustion.
A)35.6 kJ mol-1
B)686.2 kJ mol-1
C)1681 kJ mol-1
D)1886 kJ mol-1
E)2265 kJ mol-1
A)35.6 kJ mol-1
B)686.2 kJ mol-1
C)1681 kJ mol-1
D)1886 kJ mol-1
E)2265 kJ mol-1

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
79
The standard heat of combustion for naphthalene, C10H8(s), is -5156.8 kJ mol-1. Using this information and the standard enthalpies of formation, H°f: H2O(l)= -285.9 kJ mol-1; CO2(g)= ?393.5 kJ mol-1, calculate the standard enthalpy of formation of C10H8(s), in kJ mol-1.Hint: Write out an equation and remember products minus reactants for your calculations.
A)+78.2 kJ
B)+935.9 kJ
C)-1065.4 kJ
D)+3619.7 kJ
E)-10235.4 kJ
A)+78.2 kJ
B)+935.9 kJ
C)-1065.4 kJ
D)+3619.7 kJ
E)-10235.4 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
80
Complete combustion of hydrocarbons or compounds with only C, H, and O gives CO2 and H2O as the only products. If carried out under standard conditions, the CO2 is a gas and the H2O is a liquid.Given these standard enthalpies of combustion: 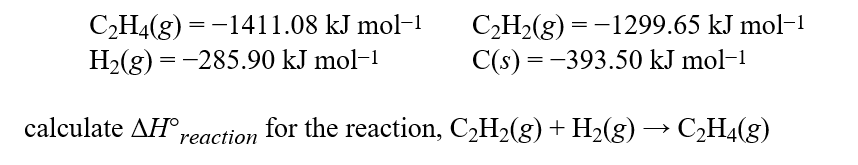 Hint: Remember it is always products minus reactants when performing enthalpy calculations.
Hint: Remember it is always products minus reactants when performing enthalpy calculations.
A)-174.47 kJ
B)+397.33 kJ
C)-961.47 kJ
D)-2424.83 kJ
E)-2996.63 kJ
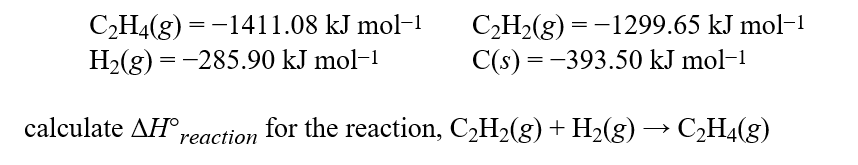 Hint: Remember it is always products minus reactants when performing enthalpy calculations.
Hint: Remember it is always products minus reactants when performing enthalpy calculations.A)-174.47 kJ
B)+397.33 kJ
C)-961.47 kJ
D)-2424.83 kJ
E)-2996.63 kJ

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck



