Deck 6: Oxidation-Reduction Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/175
Play
Full screen (f)
Deck 6: Oxidation-Reduction Reactions
1
What is the oxidation number of sulfur in the S2O82 ion?
A)-2
B)+1
C)+3
D)+5
E)+7
A)-2
B)+1
C)+3
D)+5
E)+7
+7
2
What is the oxidation number of sodium in Na2O2?
A)-2
B)-1
C)+1
D)+2
E)+4
A)-2
B)-1
C)+1
D)+2
E)+4
+1
3
What is the oxidation number of boron in Na2B4O7?
A)-3
B)+1
C)+5
D)+3
E)+6
A)-3
B)+1
C)+5
D)+3
E)+6
+3
4
What is the oxidation number of cobalt in Co2(SO4)3?
A)+3
B)+6
C)+9
D)+15
E)+30
A)+3
B)+6
C)+9
D)+15
E)+30

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
5
What is the oxidation number of sulfur in Rb2S2O4?
A)-2
B)+1
C)+3
D)+5
E)+6
A)-2
B)+1
C)+3
D)+5
E)+6

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
6
What is the oxidation number of carbon in K2C2O4?
A)0
B)-4
C)+3
D)+4
E)+6
A)0
B)-4
C)+3
D)+4
E)+6

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
7
What is the oxidation number of vanadium in (NH4)3VO4?
A)+2
B)+3
C)+5
D)+6
E)+7
A)+2
B)+3
C)+5
D)+6
E)+7

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
8
What is the oxidation number of nitrogen in (NH4)2SO4?
A)-3
B)-2
C)+1
D)+2
E)+3
A)-3
B)-2
C)+1
D)+2
E)+3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
9
What is the oxidation number of each Cu atom in [Cu2Cl6]2-?
A)+5
B)+4
C)+3
D)+2
E)+1
A)+5
B)+4
C)+3
D)+2
E)+1

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
10
What is the oxidation number of the arsenic atom in the AsO43 ion?
A)+1
B)+3
C)+4
D)+5
E)+6
A)+1
B)+3
C)+4
D)+5
E)+6

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
11
Which statement is true concerning an oxidation-reduction reaction?
A)The reactant which is reduced is the reducing agent.
B)The reactant which is oxidized is the reducing agent.
C)The reactant which gains electrons is the reducing agent.
D)The reactant which loses electrons is the oxidizing agent.
E)None of the statements, A-D, is true.
A)The reactant which is reduced is the reducing agent.
B)The reactant which is oxidized is the reducing agent.
C)The reactant which gains electrons is the reducing agent.
D)The reactant which loses electrons is the oxidizing agent.
E)None of the statements, A-D, is true.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
12
Which statement is true concerning an oxidation-reduction reaction?
A)The reactant which is reduced is the oxidizing agent.
B)The reactant which is oxidized is the oxidizing reagent.
C)The reactant which gains electrons is the reducing agent.
D)The reactant which loses electrons is the oxidizing agent.
E)None of the statements, A-D, is true.
A)The reactant which is reduced is the oxidizing agent.
B)The reactant which is oxidized is the oxidizing reagent.
C)The reactant which gains electrons is the reducing agent.
D)The reactant which loses electrons is the oxidizing agent.
E)None of the statements, A-D, is true.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
13
In a chemical reaction, one of the reactants is MnO2. It is transformed into MnSO4. What is the change in the oxidation number of the manganese?
A)There is no change in oxidation number.
B)The oxidation number increases by one unit.
C)The oxidation number increases by two units.
D)The oxidation number decreases by one unit.
E)The oxidation number decreases by two units.
A)There is no change in oxidation number.
B)The oxidation number increases by one unit.
C)The oxidation number increases by two units.
D)The oxidation number decreases by one unit.
E)The oxidation number decreases by two units.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
14
The CrO42- ion was involved in a chemical reaction, during which it is transformed into Cr3+ ions. What is the change in oxidation number of the chromium atom?
A)The oxidation number increases by 5 units.
B)The oxidation number decreases by 5 units.
C)The oxidation number increases by 1 unit.
D)The oxidation number decreases by 3 units.
E)The oxidation number decrease by 4 units.
A)The oxidation number increases by 5 units.
B)The oxidation number decreases by 5 units.
C)The oxidation number increases by 1 unit.
D)The oxidation number decreases by 3 units.
E)The oxidation number decrease by 4 units.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following involves oxidation?
A)Ba2+(aq)+ CrO42-(aq) BaCrO4(s)
B)2 H+(aq)+ CO32-(aq) H2O(l)+ CO2(g)
C)Fe3+(aq) Fe2+(aq)
D)MnO2(s) MnO4-(aq)
E)2 CrO42-(aq)+ 2 H+(aq) Cr2O72-(aq)+ H2O(l)
A)Ba2+(aq)+ CrO42-(aq) BaCrO4(s)
B)2 H+(aq)+ CO32-(aq) H2O(l)+ CO2(g)
C)Fe3+(aq) Fe2+(aq)
D)MnO2(s) MnO4-(aq)
E)2 CrO42-(aq)+ 2 H+(aq) Cr2O72-(aq)+ H2O(l)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following involves reduction?
A)Ba2+(aq)+ CrO42-(aq) BaCrO4(s)
B)2 H+(aq)+ CO32-(aq) H2O(l)+ CO2(g)
C)CrO42-(aq) Cr3+(aq)
D)MnO2(s) MnO4-(aq)
E)2 CrO42-(aq)+ 2 H+(aq) Cr2O72-(aq)+ H2O(l)
A)Ba2+(aq)+ CrO42-(aq) BaCrO4(s)
B)2 H+(aq)+ CO32-(aq) H2O(l)+ CO2(g)
C)CrO42-(aq) Cr3+(aq)
D)MnO2(s) MnO4-(aq)
E)2 CrO42-(aq)+ 2 H+(aq) Cr2O72-(aq)+ H2O(l)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following involves oxidation?
A)Ca2+(aq)+ CO32-(aq) CaCO3(s)
B)2 H+(aq)+ SO32-(aq) H2O(l)+ SO2(g)
C)VO43-(aq) VO2+(aq)
D)CrO2- (aq) CrO42-(aq)
E)2 S2O72-(aq)+ H2O(l) 2 SO42-(aq)+ 2 H+(aq)
A)Ca2+(aq)+ CO32-(aq) CaCO3(s)
B)2 H+(aq)+ SO32-(aq) H2O(l)+ SO2(g)
C)VO43-(aq) VO2+(aq)
D)CrO2- (aq) CrO42-(aq)
E)2 S2O72-(aq)+ H2O(l) 2 SO42-(aq)+ 2 H+(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following involves reduction?
A)Ca2+(aq)+ CO32-(aq) CaCO3(s)
B)2 H+(aq)+ SO32-(aq) H2O(l)+ SO2(g)
C)VO43-(aq) VO2+(aq)
D)CrO2- (aq) CrO42-(aq)
E)2 S2O72-(aq)+ H2O(l) 2 SO42-(aq)+ 2 H+(aq)
A)Ca2+(aq)+ CO32-(aq) CaCO3(s)
B)2 H+(aq)+ SO32-(aq) H2O(l)+ SO2(g)
C)VO43-(aq) VO2+(aq)
D)CrO2- (aq) CrO42-(aq)
E)2 S2O72-(aq)+ H2O(l) 2 SO42-(aq)+ 2 H+(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
19
What is the change in the oxidation number of chromium in the following? K2Cr2O7 s Cr2(SO4)3
A)increase by three units
B)increase by four units
C)decrease by two units
D)decrease by three units
E)decrease by four units
A)increase by three units
B)increase by four units
C)decrease by two units
D)decrease by three units
E)decrease by four units

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
20
What is the change in the oxidation number of carbon in the following?
CO CO2
A)increase by two units
B)increase by three units
C)increase by four units
D)decrease by three units
E)decrease by four units
CO CO2
A)increase by two units
B)increase by three units
C)increase by four units
D)decrease by three units
E)decrease by four units

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
21
What is the change in the oxidation number of nitrogen in the following?
HNO3 NO
A)increase by three units
B)increase by four units
C)decrease by two units
D)decrease by three units
E)decrease by four units
HNO3 NO
A)increase by three units
B)increase by four units
C)decrease by two units
D)decrease by three units
E)decrease by four units

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
22
What is the change in the oxidation number of manganese in the following? KMnO4 MnSO4
A)increase by two units
B)increase by three units
C)decrease by four units
D)decrease by five units
E)decrease by six units
A)increase by two units
B)increase by three units
C)decrease by four units
D)decrease by five units
E)decrease by six units

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
23
What is the change in the oxidation number of sulfur in the following?
K2SO3 K2SO4
A)decrease by three units
B)decrease by four units
C)increase by two units
D)increase by three units
E)increase by four units
K2SO3 K2SO4
A)decrease by three units
B)decrease by four units
C)increase by two units
D)increase by three units
E)increase by four units

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
24
What is the change in the oxidation number of iodine in the following?
KI KIO3
A)decrease by three units
B)decrease by four units
C)increase by six units
D)increase by five units
E)increase by four units
KI KIO3
A)decrease by three units
B)decrease by four units
C)increase by six units
D)increase by five units
E)increase by four units

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
25
What is the change in the oxidation number of carbon in the following? K2C2O4 K2CO3
A)decrease by one unit
B)decrease by two units
C)decrease by three units
D)increase by one unit
E)increase by two units
A)decrease by one unit
B)decrease by two units
C)decrease by three units
D)increase by one unit
E)increase by two units

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
26
In the equation, C4H10(l)+ Cr2O72-(aq) + H+(aq) H6C4O4(s)+ Cr3+(aq)+ H2O(l), the change in the oxidation number of the chromium atom is
A)a decrease by six units.
B)a decrease by three units.
C)an increase by three units.
D)an increase by five units.
E)an increase by eight units.
A)a decrease by six units.
B)a decrease by three units.
C)an increase by three units.
D)an increase by five units.
E)an increase by eight units.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
27
In the equation, CrO42-(aq) + H2O(l) CrO2-(aq)+ OH-(aq), the change in the oxidation number of the chromium atom is
A)decrease by six units.
B)decrease by three units.
C)increase by three units.
D)increase by five units.
E)increase by eight units.
A)decrease by six units.
B)decrease by three units.
C)increase by three units.
D)increase by five units.
E)increase by eight units.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
28
The sulfite ion (SO32-)was involved in a chemical reaction in which it underwent oxidation. Based on the change in oxidation numbers, which of the species listed below is a possible oxidation product of the reaction?
A)S2O3 2-(aq)
B)SO2(g)
C)S2-(aq)
D)S(s)
E)SO42-(aq)
A)S2O3 2-(aq)
B)SO2(g)
C)S2-(aq)
D)S(s)
E)SO42-(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
29
The nitrogen molecule, (N2), was involved in a chemical reaction in which it underwent reduction. Based on the change in oxidation numbers, which of the species listed below is a possible product of the reaction?
A)N2H4
B)NO
C)NO2-
D)NO2
E)NO3-
A)N2H4
B)NO
C)NO2-
D)NO2
E)NO3-

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
30
The ClO2 molecule was involved in a chemical reaction in which it underwent oxidation. Based on the change in oxidation numbers, which of the species listed below is a possible product of the reaction?
A)ClO-
B)Cl-
C)ClO2-
D)Cl2
E)ClO3-
A)ClO-
B)Cl-
C)ClO2-
D)Cl2
E)ClO3-

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
31
The NO molecule underwent oxidation during a chemical reaction. Which one of the species listed below is a possible product of the reaction?
A)N2H4
B)NH3
C)N2
D)NO2-
E)NH2OH
A)N2H4
B)NH3
C)N2
D)NO2-
E)NH2OH

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
32
The following unbalanced equation describes the production of metallic iron: Fe2O3 + C Fe + CO2 What is the change in oxidation number for the carbon atom?
A)decreases by 1
B)increases by 2
C)increases by 3
D)increases by 4
E)decreases by 5
A)decreases by 1
B)increases by 2
C)increases by 3
D)increases by 4
E)decreases by 5

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
33
Given the following chemical equation: SiO2 + CaCO3 CaSiO3 + CO2 What is the change in oxidation number for silicon atom?
A)There is no change.
B)decreases by 1
C)increases by 2
D)decreases by 3
E)increases by 4
A)There is no change.
B)decreases by 1
C)increases by 2
D)decreases by 3
E)increases by 4

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the redox equation below: C4H10(g)+ Cr2O72(aq)+ H+(aq) H6C4O4(aq)+ Cr3+(aq)+ H2O(l)The reducing agent is
A)C4H10(g)
B)Cr2O72(aq)
C)H+(aq)
D)H6C4O4(aq)
E)Cr3+(aq)
A)C4H10(g)
B)Cr2O72(aq)
C)H+(aq)
D)H6C4O4(aq)
E)Cr3+(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the unbalanced redox equation , C4H10(l)+ Cr2O72-(aq) + H+(aq) H6C4O4(s)+ Cr3+(aq)+ H2O(l)The oxidizing agent is
A)C4H10(l)
B)Cr2O72-(aq)
C)H+(aq)
D)H6C4O4(s)
E)Cr3+(aq)
A)C4H10(l)
B)Cr2O72-(aq)
C)H+(aq)
D)H6C4O4(s)
E)Cr3+(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the unbalanced redox equation, VO43-(aq)+ SO2(g)+ 2 H+(aq) VO2+(aq)+ SO42-(aq)+ H2O(l)The reducing agent is
A)VO43-(aq).
B)VO2+(aq).
C)H+(aq).
D)SO2(g).
E)SO42-(aq).
A)VO43-(aq).
B)VO2+(aq).
C)H+(aq).
D)SO2(g).
E)SO42-(aq).

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the redox equation,
2VO43-(aq)+ SO2(g)+ 8H+(aq) 2VO2+(aq)+ SO42-(aq)+ 4H2O(l) The oxidizing agent is
A)VO43-(aq)
B)VO2+(aq)
C)H+(aq)
D)SO2(g)
E)SO42-(aq)
2VO43-(aq)+ SO2(g)+ 8H+(aq) 2VO2+(aq)+ SO42-(aq)+ 4H2O(l) The oxidizing agent is
A)VO43-(aq)
B)VO2+(aq)
C)H+(aq)
D)SO2(g)
E)SO42-(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the redox equation,
2VO43-(aq)+ SO2(g)+ 8H+(aq) 2VO2+(aq)+ SO42-(aq)+ 4H2O(l)
Which of the following is correct?Hint: Write out the oxidation numbers for each element in the equation then compare.
A)VO43-(aq)is the oxidizing agent as the oxidation number of O decreases by 3.
B)VO2+(aq)is the reducing agent as the oxidation number of V increases by 1, causing the oxidation number of S to increase by 1 also.
C)H+(aq)is the reducing agent, its oxidation number increases from 1 to 2, as it must have lost one electron.
D)VO43-(aq)is the oxidizing agent, as the oxidation number of V decreases by 1, suggesting it must have accepted an electron.
E)SO42-(aq)is the oxidizing agent and the product formed from the reduction of SO2(g).
2VO43-(aq)+ SO2(g)+ 8H+(aq) 2VO2+(aq)+ SO42-(aq)+ 4H2O(l)
Which of the following is correct?Hint: Write out the oxidation numbers for each element in the equation then compare.
A)VO43-(aq)is the oxidizing agent as the oxidation number of O decreases by 3.
B)VO2+(aq)is the reducing agent as the oxidation number of V increases by 1, causing the oxidation number of S to increase by 1 also.
C)H+(aq)is the reducing agent, its oxidation number increases from 1 to 2, as it must have lost one electron.
D)VO43-(aq)is the oxidizing agent, as the oxidation number of V decreases by 1, suggesting it must have accepted an electron.
E)SO42-(aq)is the oxidizing agent and the product formed from the reduction of SO2(g).

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
39
The following unbalanced equation describes the production of metallic iron: Fe2O3 + C Fe + CO2 Which is true?Hint: Remember the reducing agent is oxidized and the oxidizing agent is reduced.
A)Fe2O3 is the reducing agent, since it is reduced, while C is the oxidizing agent, since it oxidizes Fe2O3.
B)Fe2O3 is the reducing agent, while Fe is the oxidizing agent, as it oxidizes C to CO2.
C)Fe is the reducing agent, since it reduces C, while C is the oxidizing agent.
D)Fe2O3 is the oxidizing agent, while C is the reducing agent, since it reduces Fe2O3.
E)Fe2O3 is the reducing agent, while CO2 is the oxidizing agent.
A)Fe2O3 is the reducing agent, since it is reduced, while C is the oxidizing agent, since it oxidizes Fe2O3.
B)Fe2O3 is the reducing agent, while Fe is the oxidizing agent, as it oxidizes C to CO2.
C)Fe is the reducing agent, since it reduces C, while C is the oxidizing agent.
D)Fe2O3 is the oxidizing agent, while C is the reducing agent, since it reduces Fe2O3.
E)Fe2O3 is the reducing agent, while CO2 is the oxidizing agent.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
40
Balance the half-reaction, H2S S8, taking place in acidic media. How many electrons are needed to balance the half-reaction?Hint: Remember the coefficients when balancing half-reactions, and look carefully at the oxidation numbers.
A)12 electrons, left side
B)16 electrons, right side
C)14 electrons, left side
D)6 electrons, right side
E)8 electrons, right side
A)12 electrons, left side
B)16 electrons, right side
C)14 electrons, left side
D)6 electrons, right side
E)8 electrons, right side

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
41
Balance the half-reaction, NO3 NH4+, taking place in acidic media. How many electrons are needed to balance the half-reaction?Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)2 electrons, left side
B)3 electrons, right side
C)4 electrons, left side
D)8 electrons, left side
E)8 electrons, right side
A)2 electrons, left side
B)3 electrons, right side
C)4 electrons, left side
D)8 electrons, left side
E)8 electrons, right side

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
42
Balance the half-reaction, Cl2O7 HClO, taking place in acidic media. How many electrons are needed to balance the half-reaction?Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)2 electrons, left side
B)3 electrons, right side
C)12 electrons, left side
D)6 electrons, right side
E)8 electrons, left side
A)2 electrons, left side
B)3 electrons, right side
C)12 electrons, left side
D)6 electrons, right side
E)8 electrons, left side

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
43
Balance the half-reaction, C2H6O HC2H3O2, taking place in acidic media. How many electrons are needed to balance the charge?Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)2 electrons, left side
B)3 electrons, left side
C)4 electrons, right side
D)6 electrons, right side
E)8 electrons, right side
A)2 electrons, left side
B)3 electrons, left side
C)4 electrons, right side
D)6 electrons, right side
E)8 electrons, right side

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
44
Balance the half-reaction, C8H10 C8H4O42, taking place in basic media. How many electrons are needed to balance the half-reaction?Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)4 electrons, left side
B)8 electrons, right side
C)8 electrons, left side
D)12 electrons, left side
E)12 electrons, right side
A)4 electrons, left side
B)8 electrons, right side
C)8 electrons, left side
D)12 electrons, left side
E)12 electrons, right side

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
45
Complete the balancing of the following half-reaction, taking place in basic media. How many electrons are needed to balance the half-reaction?
Br(aq) BrO3(aq) Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)2 electrons, left side
B)2 electrons, right side
C)4 electrons, right side
D)6 electrons, right side
E)6 electrons, left side
Br(aq) BrO3(aq) Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)2 electrons, left side
B)2 electrons, right side
C)4 electrons, right side
D)6 electrons, right side
E)6 electrons, left side

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
46
Complete the balancing of the following half-reaction, taking place in basic media. How many electrons are needed to balance the half-reaction?
Cr(OH)4(aq) CrO42(aq)Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)1 electron, on the left side
B)2 electrons, on the right side
C)3 electrons, on the right side
D)3 electrons, on the left side
E)4 electrons, on the right side
Cr(OH)4(aq) CrO42(aq)Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)1 electron, on the left side
B)2 electrons, on the right side
C)3 electrons, on the right side
D)3 electrons, on the left side
E)4 electrons, on the right side

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
47
Complete the balancing of the following half-reaction, taking place in basic media. How many hydroxide ions are needed to balance the half-reaction?Br(aq)s BrO3(aq)Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)2 hydroxide ions, on the left side
B)4 hydroxide ions, on the left side
C)4 hydroxide ions, on the right side
D)6 hydroxide ions, on the left side
E)6 hydroxide ions, on the right side
A)2 hydroxide ions, on the left side
B)4 hydroxide ions, on the left side
C)4 hydroxide ions, on the right side
D)6 hydroxide ions, on the left side
E)6 hydroxide ions, on the right side

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
48
Complete the balancing of the following half-reaction, taking place in basic media. How many hydroxide ions are needed to balance the half-reaction? Cr(OH)4(aq) CrO42(aq)Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A)1 hydroxide ion, on the left side
B)3 hydroxide ions, on the right side
C)3 hydroxide ions, on the left side
D)4 hydroxide ions, on the left side
E)4 hydroxide ions, on the right side
A)1 hydroxide ion, on the left side
B)3 hydroxide ions, on the right side
C)3 hydroxide ions, on the left side
D)4 hydroxide ions, on the left side
E)4 hydroxide ions, on the right side

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
49
After balancing the following equation for the reaction in acidic media,
Fe2+(aq)+ Cr2O72(aq)+ H+(aq) Cr3+(aq)+ Fe3+(aq)+ H2O(l)what is the sum of ALL the coefficients in the equation?(Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)6
B)22
C)30
D)32
E)36
Fe2+(aq)+ Cr2O72(aq)+ H+(aq) Cr3+(aq)+ Fe3+(aq)+ H2O(l)what is the sum of ALL the coefficients in the equation?(Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)6
B)22
C)30
D)32
E)36

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
50
After balancing the following equation for the reaction in acidic media,
MnO4-(aq)+ H2C2O4(aq)+ H+(aq) Mn2+(aq)+ CO2(g)+ H2O(l)what is the sum of ALL the coefficients in the equation?(Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)29
B)30
C)31
D)32
E)33
MnO4-(aq)+ H2C2O4(aq)+ H+(aq) Mn2+(aq)+ CO2(g)+ H2O(l)what is the sum of ALL the coefficients in the equation?(Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)29
B)30
C)31
D)32
E)33

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
51
After balancing the following equation for the reaction in acidic media,
H+ + HSO3-(aq)+ MnO4-(aq) MnO2(s)+ HSO4-(aq)+ H2O what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)7
B)9
C)13
D)15
E)19
H+ + HSO3-(aq)+ MnO4-(aq) MnO2(s)+ HSO4-(aq)+ H2O what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)7
B)9
C)13
D)15
E)19

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
52
After balancing the following equation for the reaction in basic media,
OH- + Bi2O3(s)+ OCl-(aq) Cl-(aq)+ BiO3-(aq)+ H2O what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)6
B)8
C)9
D)10
E)14
OH- + Bi2O3(s)+ OCl-(aq) Cl-(aq)+ BiO3-(aq)+ H2O what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)6
B)8
C)9
D)10
E)14

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
53
After balancing the following equation for the reaction in basic media,
H2O + CrO42-(aq)+ Br-(aq) CrO2-(aq)+ BrO3-(aq)+ OH- what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)6
B)8
C)9
D)10
E)14
H2O + CrO42-(aq)+ Br-(aq) CrO2-(aq)+ BrO3-(aq)+ OH- what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)6
B)8
C)9
D)10
E)14

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
54
After balancing the following equation for the reaction in basic media,
I-(aq)+ MnO4-(aq) I2(aq)+ MnO2(s)what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)6
B)13
C)17
D)25
E)28
I-(aq)+ MnO4-(aq) I2(aq)+ MnO2(s)what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
A)6
B)13
C)17
D)25
E)28

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
55
Zinc metal reacts with perchloric acid solution to produce aqueous zinc perchlorate and hydrogen gas, which escapes. The species being oxidized in this reaction is
A)HClO4(aq).
B)H2(g).
C)Zn2+(aq).
D)Zn(s).
E)Zn(ClO4)2(aq).
A)HClO4(aq).
B)H2(g).
C)Zn2+(aq).
D)Zn(s).
E)Zn(ClO4)2(aq).

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
56
Sodium bicarbonate, (NaHCO3), in solution reacts with hydrochloric acid solution to produce aqueous sodium chloride, carbon dioxide gas, and water. The species being reduced in this reaction is Hint: Oxidation is a loss of electrons and reduction is a gain of electrons.
A)HCO3- (aq).
B)CO2(g).
C)HCl(aq).
D)Cl-(aq).
E)none of the above.
A)HCO3- (aq).
B)CO2(g).
C)HCl(aq).
D)Cl-(aq).
E)none of the above.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
57
Zinc metal reacts with nitric acid solution to produce aqueous zinc nitrate, ammonium nitrate (in solution), and water. The species being oxidized in this reaction is
A)HNO3(aq).
B)NH4+(aq).
C)Zn2+(aq).
D)Zn(s).
E)Zn(NO3)2(aq).
A)HNO3(aq).
B)NH4+(aq).
C)Zn2+(aq).
D)Zn(s).
E)Zn(NO3)2(aq).

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
58
Zinc metal reacts with nitric acid solution to produce zinc nitrate (in solution), ammonium nitrate (in solution), and water. The species being reduced in this reaction is
A)HNO3(aq).
B)NH4+(aq).
C)Zn2+(aq).
D)Zn(s).
E)Zn(NO3)2(aq).
A)HNO3(aq).
B)NH4+(aq).
C)Zn2+(aq).
D)Zn(s).
E)Zn(NO3)2(aq).

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
59
Nitric acid reacts with copper metal according to the unbalanced equation,
Cu(s)+ HNO3(aq) Cu(NO3)2(aq)+ NO2(g)+ H2O Which is correct?
A)The nitrate ion is the reducing agent, and it is reduced to water.
B)The product of the reduction reaction is NO2.
C)The product of the reduction reaction is Cu(NO3)2.
D)Copper is the oxidizing agent, and it is reduced to Cu(NO3)2.
E)Both water and copper are reduced.
Cu(s)+ HNO3(aq) Cu(NO3)2(aq)+ NO2(g)+ H2O Which is correct?
A)The nitrate ion is the reducing agent, and it is reduced to water.
B)The product of the reduction reaction is NO2.
C)The product of the reduction reaction is Cu(NO3)2.
D)Copper is the oxidizing agent, and it is reduced to Cu(NO3)2.
E)Both water and copper are reduced.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
60
Magnesium metal reacts with aqueous sulfuric acid solution to produce aqueous magnesium sulfate and hydrogen gas. In the course of the reaction, which element undergoes an increase in oxidation number?
A)hydrogen
B)magnesium
C)oxygen
D)sulfur
E)both magnesium and hydrogen
A)hydrogen
B)magnesium
C)oxygen
D)sulfur
E)both magnesium and hydrogen

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
61
The reaction of potassium metal and which one of the following ions could be used to produce the most electrical current, per mole of potassium? Hint: Look at the activity series.
A)Ag+
B)H+
C)Mn+
D)Sr2+
E)Rb+
A)Ag+
B)H+
C)Mn+
D)Sr2+
E)Rb+

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
62
The most reactive metals are those of
A)Group IA (Group 1).
B)Group IIA (Group 2).
C)Group IIIA (Group 13).
D)Group IB (Group 11).
E)Group IIB (Group 12).
A)Group IA (Group 1).
B)Group IIA (Group 2).
C)Group IIIA (Group 13).
D)Group IB (Group 11).
E)Group IIB (Group 12).

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
63
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Based on this list, which element below would undergo oxidation most readily?
A)Ag
B)Al
C)Cu
D)Cd
E)Zn
A)Ag
B)Al
C)Cu
D)Cd
E)Zn

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
64
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Based on this list, which element below would undergo oxidation most readily?
A)Mg
B)Al
C)Cu
D)Cd
E)Zn
A)Mg
B)Al
C)Cu
D)Cd
E)Zn

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
65
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Based on this list, which element below would undergo oxidation least readily?
A)Mg
B)Al
C)Cu
D)Cd
E)Zn
A)Mg
B)Al
C)Cu
D)Cd
E)Zn

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
66
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Based on this list, which element below would undergo oxidation least readily?
A)Ag
B)Al
C)Cu
D)Cd
E)Zn
A)Ag
B)Al
C)Cu
D)Cd
E)Zn

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
67
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Based on this list, which element below would react most readily with a 0.10 M aqueous solution of hydrofluoric acid?
A)Ag
B)Sn
C)Cu
D)Cd
E)Zn
A)Ag
B)Sn
C)Cu
D)Cd
E)Zn

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
68
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Based on this list, which element below would react most readily with a 0.10 M aqueous solution of hydrochloric acid?
A)Au
B)Sn
C)Cu
D)Al
E)Mg
A)Au
B)Sn
C)Cu
D)Al
E)Mg

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
69
Three metallic elements, copper, gold and zinc, can be distinguished from one another on the basis of how they react with two strong acids, HNO3(aq)and HCl(aq). Which set below, using the abbreviations R (for reaction occurs)and NR (for no reaction)correctly describes what occurs? 


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
70
Three metallic elements, copper, magnesium and gold, can be distinguished from one another on the basis of how they react with two strong acids, HNO3(aq)and HCl(aq). Which set below, using the abbreviations R (for reaction occurs)and NR (no reaction)correctly describes what occurs? 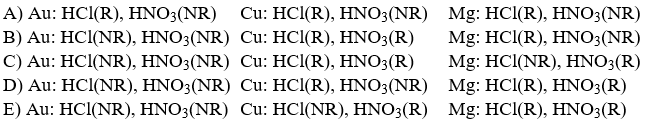
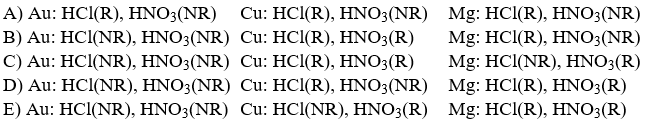

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
71
The least reactive metals are those of
A)Group IA (Group 1)
B)Group IIA (Group 2)
C)Group IIIA (Group 13)
D)Group IB (Group 11)
E)Group IIB (Group 12)
A)Group IA (Group 1)
B)Group IIA (Group 2)
C)Group IIIA (Group 13)
D)Group IB (Group 11)
E)Group IIB (Group 12)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
72
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Which reaction below does not occur spontaneously upon mixing the reagents shown?
A)Cd(s)+ Al3+(aq) Cd2+(aq)+ Al(s)
B)Cd(s)+ Cu2+(aq) Cd2+(aq)+ Cu(s)
C)Zn(s)+ Cu2+(aq) Zn2+(aq)+ Cu(s)
D)Al(s)+ Ag+(aq) Al3+(aq)+ Ag(s)
E)Cu(s)+ Au3+(aq) Cu2+(aq)+ Au(s)
A)Cd(s)+ Al3+(aq) Cd2+(aq)+ Al(s)
B)Cd(s)+ Cu2+(aq) Cd2+(aq)+ Cu(s)
C)Zn(s)+ Cu2+(aq) Zn2+(aq)+ Cu(s)
D)Al(s)+ Ag+(aq) Al3+(aq)+ Ag(s)
E)Cu(s)+ Au3+(aq) Cu2+(aq)+ Au(s)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
73
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Which reaction below does not occur spontaneously upon mixing the reagents shown?
A)Al(s)+ Cd2+(aq) Al3+(aq)+ Cd(s)
B)Mg(s)+ Cu2+(aq) Mg2+(aq)+ Cu(s)
C)Zn(s)+ Ag+(aq) Zn2+(aq)+ Ag(s)
D)Zn(s)+ Al3+(aq) Zn2+(aq)+ Al(s)
E)Na(s)+ Au3+(aq) Na+(aq)+ Au(s)
A)Al(s)+ Cd2+(aq) Al3+(aq)+ Cd(s)
B)Mg(s)+ Cu2+(aq) Mg2+(aq)+ Cu(s)
C)Zn(s)+ Ag+(aq) Zn2+(aq)+ Ag(s)
D)Zn(s)+ Al3+(aq) Zn2+(aq)+ Al(s)
E)Na(s)+ Au3+(aq) Na+(aq)+ Au(s)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
74
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Which reaction below occurs spontaneously upon mixing the reagents shown?
A)Sn(s)+ Zn2+(aq) Sn2+(aq)+ Zn(s)
B)Ag(s)+ Mg2+(aq) Ag+(aq)+ Mg(s)
C)Zn(s)+ Au3+(aq) Zn2+(aq)+ Au(s)
D)Ag(s)+ Zn2+(aq) Ag+(aq)+ Zn(s)
E)Sn(s)+ Al3+(aq) Sn2+(aq)+ Al(s)
A)Sn(s)+ Zn2+(aq) Sn2+(aq)+ Zn(s)
B)Ag(s)+ Mg2+(aq) Ag+(aq)+ Mg(s)
C)Zn(s)+ Au3+(aq) Zn2+(aq)+ Au(s)
D)Ag(s)+ Zn2+(aq) Ag+(aq)+ Zn(s)
E)Sn(s)+ Al3+(aq) Sn2+(aq)+ Al(s)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
75
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Which reaction below occurs spontaneously upon mixing the reagents shown?
A)Ag(s)+ Zn2+(aq) Ag+(aq)+ Zn(s)
B)Ag(s)+ Cd2+(aq) Ag+(aq)+ Cd(s)
C)Zn(s)+ Mg2+(aq) Zn2+(aq)+ Mg(s)
D)Ag(s)+ Mg2+(aq) Ag+(aq)+ Mg(s)
E)Sn(s)+ Cu2+(aq) Sn2+(aq)+ Cu(s)
A)Ag(s)+ Zn2+(aq) Ag+(aq)+ Zn(s)
B)Ag(s)+ Cd2+(aq) Ag+(aq)+ Cd(s)
C)Zn(s)+ Mg2+(aq) Zn2+(aq)+ Mg(s)
D)Ag(s)+ Mg2+(aq) Ag+(aq)+ Mg(s)
E)Sn(s)+ Cu2+(aq) Sn2+(aq)+ Cu(s)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
76
Which element would react most rapidly with water?
A)Ag
B)K
C)Cu
D)Na
E)Rb
A)Ag
B)K
C)Cu
D)Na
E)Rb

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
77
Which element would react most rapidly with water?
A)K
B)Cd
C)Cs
D)Na
E)Zn
A)K
B)Cd
C)Cs
D)Na
E)Zn

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
78
Which element would be the least likely to react, if at all, with water?
A)Ca
B)Al
C)Cu
D)Na
E)Zn
A)Ca
B)Al
C)Cu
D)Na
E)Zn

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
79
A partial activity series is: Au < Ag < Cu < H2 < Sn < Cd < Fe < Mn. Which combination of metals is likely to be produced when each of the following compounds is made to react with solid Cd metal:Cd(NO3)2(aq); Fe(NO3)2(aq); HNO3(aq); Cu(NO3)2(aq); Sn(NO3)2(aq)? Hint: In the partial activity series above, Mn is the most reactive and Au is the least reactive.
A)Cd, Fe
B)Cu, Cd
C)Cu, Fe
D)Fe, Sn
E)Sn, Cu
A)Cd, Fe
B)Cu, Cd
C)Cu, Fe
D)Fe, Sn
E)Sn, Cu

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
80
A partial activity series is: Au < Ag < Cu < H2 < Sn < Cd < Fe < Mn. Which metal(s)is/are NOT likely to be produced when each of the following compounds is made to react with Cd metal:Cd(NO3)2(aq); Fe(NO3)2(aq); HNO3(aq); Cu(NO3)2(aq); Sn(NO3)2(aq); AgNO3(aq)? Hint: In the partial activity series above, Mn is the most reactive and Au is the least reactive.
A)Cu, Sn
B)Sn, Ag
C)Cu
D)Fe, Cd
E)Ag, Cu
A)Cu, Sn
B)Sn, Ag
C)Cu
D)Fe, Cd
E)Ag, Cu

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck



