Deck 17: Electron Transfer Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/76
Play
Full screen (f)
Deck 17: Electron Transfer Reactions
1
Use oxidation numbers to show what is being oxidized and what is being reduced in a redox reaction.
Oxidation is the loss of electrons from a substance. Reduction is the gain of electrons by a substance. Oxidation and reduction always occur together.
2
Balance redox reactions using the half-reaction method.
Redox reactions can be separated into two half-reactions, one for the oxidation and one for the reduction.
3
Describe galvanic cells.
Oxidation always occurs at the anode and reduction always occurs at the cathode. A galvanic cell has a spontaneous redox reaction.
4
Calculate standard cell potentials.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
5
Relate cell potential to the reaction conditions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
6
Explain the chemistry of everyday redox reactions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
7
Explain electrolytic reactions and cells.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is NOT a redox reaction?
A) 4 NH3 + 5 O2 4 NO + 6 H2O
B) 2 CO + O2 2 CO2
C) S + 2 F2 SF4
D) AgNO3 + KI AgI(s) + KNO3
E) Cl2 + 2H2O (\rarr\) 2Cl- + 2OCl- + 4H+
A) 4 NH3 + 5 O2 4 NO + 6 H2O
B) 2 CO + O2 2 CO2
C) S + 2 F2 SF4
D) AgNO3 + KI AgI(s) + KNO3
E) Cl2 + 2H2O (\rarr\) 2Cl- + 2OCl- + 4H+

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following are redox reactions?
I. 2 N2 + 3 H2 2 NH3
II. 4 Al + 3 O2 2 Al2O3
III. 2 NO2 N2O4
IV. FeCl3 (aq) + 6 NH3(aq) (\rarr\)Fe(NH3)63+ (aq) + 3 Cl- (aq)
V. Cu2+ + Zn Cu + Zn2+
A) I, IV and V
B) All but III
C) I and II
D) I, II and IV
E) I, II and V
I. 2 N2 + 3 H2 2 NH3
II. 4 Al + 3 O2 2 Al2O3
III. 2 NO2 N2O4
IV. FeCl3 (aq) + 6 NH3(aq) (\rarr\)Fe(NH3)63+ (aq) + 3 Cl- (aq)
V. Cu2+ + Zn Cu + Zn2+
A) I, IV and V
B) All but III
C) I and II
D) I, II and IV
E) I, II and V

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
10
Which elements are changing oxidation states in the following reaction?
Zn(s) + 2 MnO2(s) + H2O(l) Zn(OH)2(s) + Mn2O3(s)
A) Zn, O
B) Mn, Zn
C) H, Mn
D) O, Mn
E) H, O
Zn(s) + 2 MnO2(s) + H2O(l) Zn(OH)2(s) + Mn2O3(s)
A) Zn, O
B) Mn, Zn
C) H, Mn
D) O, Mn
E) H, O

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
11
Assign oxidation numbers to all the elements in LiAlH4.
A) Li, +1; Al, +3; H, +1
B) Li, +1; Al, -5; H, +1
C) Li, +1; Al, +3; H, -1
D) Li, +1; Al, -2; H, +1
E) Li, +1; Al, 0; H, -1
A) Li, +1; Al, +3; H, +1
B) Li, +1; Al, -5; H, +1
C) Li, +1; Al, +3; H, -1
D) Li, +1; Al, -2; H, +1
E) Li, +1; Al, 0; H, -1

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
12
Assign oxidation numbers to all the elements in KCN.
A) K, +1; C +4; N, -3
B) K, +1; C +2; N, -3
C) K, +1; C -4; N, +3
D) K, +1; C -6; N, +5
E) K, +1; C,+3; N -4
A) K, +1; C +4; N, -3
B) K, +1; C +2; N, -3
C) K, +1; C -4; N, +3
D) K, +1; C -6; N, +5
E) K, +1; C,+3; N -4

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
13
Nitrogen has many possible oxidation numbers; put the following nitrogen compounds in order of increasing oxidation number: NO2, HNO3, NO2-, NO.
A) NO2, HNO3, NO2-, NO
B) NO, HNO3, NO2-, NO2
C) HNO3, NO2, NO2-, NO
D) NO, NO2-, NO2, HNO3
E) NO2-, NO, NO2, HNO3
A) NO2, HNO3, NO2-, NO
B) NO, HNO3, NO2-, NO2
C) HNO3, NO2, NO2-, NO
D) NO, NO2-, NO2, HNO3
E) NO2-, NO, NO2, HNO3

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
14
Determine the coefficient for Sn+2 in the following balanced redox reaction.MnO4- + Sn2+ Sn4+ + Mn2+ (acidic solution)
A) 2
B) 4
C) 5
D) 6
E) 10
A) 2
B) 4
C) 5
D) 6
E) 10

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the redox reaction of permanganate and sulphur:
MnO4- + S Mn2+ + SO42- (acidic solution)If the coefficient of MnO4- is 6 in the balanced equation, what is the coefficient of H2O?
A) 2
B) 3
C) 4
D) 5
E) 6
MnO4- + S Mn2+ + SO42- (acidic solution)If the coefficient of MnO4- is 6 in the balanced equation, what is the coefficient of H2O?
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the redox reaction of nitric acid and copper:
Cu + HNO3 Cu(NO3)2 + NO (acidic solution)If the coefficient of Cu is 3 in the balanced equation, what is the coefficient of HNO3?
A) 4
B) 5
C) 7
D) 8
E) 10
Cu + HNO3 Cu(NO3)2 + NO (acidic solution)If the coefficient of Cu is 3 in the balanced equation, what is the coefficient of HNO3?
A) 4
B) 5
C) 7
D) 8
E) 10

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
17
Consider the redox reaction of triiodide and oxygen:I3- + O2 I2 + OH- (basic solution)If the coefficient of I3- is 4 in the balanced equation, what is the coefficient of OH-?
A) 2
B) 4
C) 6
D) 8
E) 10
A) 2
B) 4
C) 6
D) 8
E) 10

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
18
The following reaction occurs in a galvanic cell:
NiO2 + Cd + H2O Cd(OH)2 + Ni(OH)2 + 2 OH-Which redox process in this battery occurs at a passive electrode?
A) Cd Cd(OH)2
B) NiO2 Ni(OH)2
C) O2 4 OH-
D) H2O OH-
E) neither electrode is passive
NiO2 + Cd + H2O Cd(OH)2 + Ni(OH)2 + 2 OH-Which redox process in this battery occurs at a passive electrode?
A) Cd Cd(OH)2
B) NiO2 Ni(OH)2
C) O2 4 OH-
D) H2O OH-
E) neither electrode is passive

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
19
What is the role of the electrolyte in a galvanic cell?
A) to facilitate rapid diffusion of the redox reagents to each other
B) to facilitate electron transport though the solution
C) to complete the electrical circuit by ion transport
D) to supply the ions for precipitating redox products
E) to protect electrodes from corrosion
A) to facilitate rapid diffusion of the redox reagents to each other
B) to facilitate electron transport though the solution
C) to complete the electrical circuit by ion transport
D) to supply the ions for precipitating redox products
E) to protect electrodes from corrosion

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
20
For the reaction given, which half reaction occurs at the cathode?
NiO2 + Cd + H2O Cd(OH)2 + Ni(OH)2 + 2 OH-
A) Cd Cd(OH)2
B) NiO2 Ni(OH)2
C) Cd(OH)2 Cd
D) H2O OH-
E) There is no cathode as the cathodic reaction always occurs at the passive electrode.
NiO2 + Cd + H2O Cd(OH)2 + Ni(OH)2 + 2 OH-
A) Cd Cd(OH)2
B) NiO2 Ni(OH)2
C) Cd(OH)2 Cd
D) H2O OH-
E) There is no cathode as the cathodic reaction always occurs at the passive electrode.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
21
For the reaction given below, which half reaction occurs at the cathode?Pb(s) + PbO2(s) + 2HSO4-(aq) + 2H3O+(aq) 2PbSO4(aq) +4H2O(l)
A) Pb PbSO4
B) PbO2 PbSO4
C) HSO4- PbSO4
D) H3O+ H2O
E) PbSO4 PbO2
A) Pb PbSO4
B) PbO2 PbSO4
C) HSO4- PbSO4
D) H3O+ H2O
E) PbSO4 PbO2

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
22
For the reaction given below, identify the anode and describe what happens to the electrode as the reaction continues.
3Fe(s) + Cr2O72-(aq) + 14 H+(aq) 3Fe2+(aq) + 2 Cr+3(aq) + 7H2O(l)
A) Fe, converted to Fe2+; electrode decreases in size
B) Fe, converted to Fe2+; electrode gains mass
C) Passive electrode at which Cr2O72-(aq) is oxidized; electrode is unchanged
D) Fe electrode at which Cr+3 is oxidized; electrode is unchanged
E) Fe electrode; no change
3Fe(s) + Cr2O72-(aq) + 14 H+(aq) 3Fe2+(aq) + 2 Cr+3(aq) + 7H2O(l)
A) Fe, converted to Fe2+; electrode decreases in size
B) Fe, converted to Fe2+; electrode gains mass
C) Passive electrode at which Cr2O72-(aq) is oxidized; electrode is unchanged
D) Fe electrode at which Cr+3 is oxidized; electrode is unchanged
E) Fe electrode; no change

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
23
For the reaction given below, which half reaction occurs at the anode?
2 H2(g) + O2(g) 2 H2O(l)
A) H2 H2O
B) O2 H2O
C) H2O H2
D) H2O O2
E) H2(g) 2H+
2 H2(g) + O2(g) 2 H2O(l)
A) H2 H2O
B) O2 H2O
C) H2O H2
D) H2O O2
E) H2(g) 2H+

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
24
Consult a table of reduction potentials (Table 17-1 in the text) and determine which two metals are capable of reducing iron (II) to iron under standard conditions.
A) Ca, Sn
B) Sn, Pb
C) Al, Mg
D) Mg, Cu
E) Cd, Hg
A) Ca, Sn
B) Sn, Pb
C) Al, Mg
D) Mg, Cu
E) Cd, Hg

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
25
Platinum metal is quite resistant to oxidation as may be deduced by its reduction potential:Pt2+ + 2e Pt E° 1.2 VExamine a table of reduction potentials (Table 17-1 in the text) and determine two elements capable of oxidizing platinum under standard conditions.
A) Au, F2
B) F2, Fe
C) F2, Cl2
D) Br2, Ag
E) Mn, Au
A) Au, F2
B) F2, Fe
C) F2, Cl2
D) Br2, Ag
E) Mn, Au

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
26
Consider an electrochemical cell consisting of an Fe(s) electrode, Fe(NO3)2 electrolyte connected through a salt bridge to a Ag wire coated in AgCl(s) immersed in an aqueous KCl solution. Is the standard cell and balanced galvanic cell reaction:(If needed, refer to Table 17-1 in the text )
A) 0.259 V, Fe(s) + 3AgCl(s) Fe3+(aq) + 3Ag(s) + 3Cl-(aq)
B) 0.669 V, Fe(s) + 2AgCl(s) Fe2+(aq) + 2Ag(s) + 2Cl-(aq)
C) -0.669 V, Fe(s) + 2AgCl(s) Fe2+(aq) + 3Ag(s) + 3Cl-(aq)
D) -0.225 V, Fe(s) + 2AgCl(s) Fe2+(aq) + 2Ag(s) + 2Cl-(aq)
E) 0.669 V, Fe(s) + AgCl(s) Fe2+(aq) + Ag(s) + Cl-(aq)
A) 0.259 V, Fe(s) + 3AgCl(s) Fe3+(aq) + 3Ag(s) + 3Cl-(aq)
B) 0.669 V, Fe(s) + 2AgCl(s) Fe2+(aq) + 2Ag(s) + 2Cl-(aq)
C) -0.669 V, Fe(s) + 2AgCl(s) Fe2+(aq) + 3Ag(s) + 3Cl-(aq)
D) -0.225 V, Fe(s) + 2AgCl(s) Fe2+(aq) + 2Ag(s) + 2Cl-(aq)
E) 0.669 V, Fe(s) + AgCl(s) Fe2+(aq) + Ag(s) + Cl-(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu2+ (aq) Zn2+ (aq) + Cu (s) E° = 1.10 VIf the cell is initially at standard conditions ([Cu2+] = [Zn2+] = 1.00 M), what are the concentrations of Cu2+ and Zn2+ when the cell potential has fallen to 1.06 V?If needed, use the following equation: G° = -nFE°, E° ![<strong>Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu<sup>2+</sup><sup> </sup>(aq) \rarr Zn<sup>2+</sup><sup> </sup>(aq) + Cu (s) \quad E° = 1.10 VIf the cell is initially at standard conditions ([Cu<sup>2+</sup>] = [Zn<sup>2+</sup>] = 1.00 M), what are the concentrations of Cu<sup>2+</sup><sup> </sup>and Zn<sup>2+</sup> when the cell potential has fallen to 1.06 V?If needed, use the following equation: \Delta G° = -nFE°, E° , E = E° - , moles e<sup>-</sup> = (If needed, refer to Table 17-1in the text)</strong> A) [Cu<sup>2+</sup>]= 8.5 x 10<sup>-2</sup> M; [Zn<sup>2+</sup>]=1.91 M B) [Cu<sup>2+</sup>]= 0.94 M; [Zn<sup>2+</sup>]= 1.06 M C) [Cu<sup>2+</sup>]= 1.91 M; [Zn<sup>2+</sup>]=8.5 x 10<sup>-2</sup> M D) [Cu<sup>2+</sup>]= 0.50 M; [Zn<sup>2+</sup>]=1.50 M E) [Cu<sup>2+</sup>]= 0.90 M; [Zn<sup>2+</sup>]=1.10 M](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d433_2f97_827e_430b5f32088f_TB9687_11.jpg) , E = E° -
, E = E° - ![<strong>Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu<sup>2+</sup><sup> </sup>(aq) \rarr Zn<sup>2+</sup><sup> </sup>(aq) + Cu (s) \quad E° = 1.10 VIf the cell is initially at standard conditions ([Cu<sup>2+</sup>] = [Zn<sup>2+</sup>] = 1.00 M), what are the concentrations of Cu<sup>2+</sup><sup> </sup>and Zn<sup>2+</sup> when the cell potential has fallen to 1.06 V?If needed, use the following equation: \Delta G° = -nFE°, E° , E = E° - , moles e<sup>-</sup> = (If needed, refer to Table 17-1in the text)</strong> A) [Cu<sup>2+</sup>]= 8.5 x 10<sup>-2</sup> M; [Zn<sup>2+</sup>]=1.91 M B) [Cu<sup>2+</sup>]= 0.94 M; [Zn<sup>2+</sup>]= 1.06 M C) [Cu<sup>2+</sup>]= 1.91 M; [Zn<sup>2+</sup>]=8.5 x 10<sup>-2</sup> M D) [Cu<sup>2+</sup>]= 0.50 M; [Zn<sup>2+</sup>]=1.50 M E) [Cu<sup>2+</sup>]= 0.90 M; [Zn<sup>2+</sup>]=1.10 M](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d433_2f98_827e_cfe7754dd8fc_TB9687_11.jpg) , moles e- =
, moles e- = ![<strong>Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu<sup>2+</sup><sup> </sup>(aq) \rarr Zn<sup>2+</sup><sup> </sup>(aq) + Cu (s) \quad E° = 1.10 VIf the cell is initially at standard conditions ([Cu<sup>2+</sup>] = [Zn<sup>2+</sup>] = 1.00 M), what are the concentrations of Cu<sup>2+</sup><sup> </sup>and Zn<sup>2+</sup> when the cell potential has fallen to 1.06 V?If needed, use the following equation: \Delta G° = -nFE°, E° , E = E° - , moles e<sup>-</sup> = (If needed, refer to Table 17-1in the text)</strong> A) [Cu<sup>2+</sup>]= 8.5 x 10<sup>-2</sup> M; [Zn<sup>2+</sup>]=1.91 M B) [Cu<sup>2+</sup>]= 0.94 M; [Zn<sup>2+</sup>]= 1.06 M C) [Cu<sup>2+</sup>]= 1.91 M; [Zn<sup>2+</sup>]=8.5 x 10<sup>-2</sup> M D) [Cu<sup>2+</sup>]= 0.50 M; [Zn<sup>2+</sup>]=1.50 M E) [Cu<sup>2+</sup>]= 0.90 M; [Zn<sup>2+</sup>]=1.10 M](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d433_2f99_827e_3f1d17c3c852_TB9687_11.jpg) (If needed, refer to Table 17-1in the text)
(If needed, refer to Table 17-1in the text)
A) [Cu2+]= 8.5 x 10-2 M; [Zn2+]=1.91 M
B) [Cu2+]= 0.94 M; [Zn2+]= 1.06 M
C) [Cu2+]= 1.91 M; [Zn2+]=8.5 x 10-2 M
D) [Cu2+]= 0.50 M; [Zn2+]=1.50 M
E) [Cu2+]= 0.90 M; [Zn2+]=1.10 M
![<strong>Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu<sup>2+</sup><sup> </sup>(aq) \rarr Zn<sup>2+</sup><sup> </sup>(aq) + Cu (s) \quad E° = 1.10 VIf the cell is initially at standard conditions ([Cu<sup>2+</sup>] = [Zn<sup>2+</sup>] = 1.00 M), what are the concentrations of Cu<sup>2+</sup><sup> </sup>and Zn<sup>2+</sup> when the cell potential has fallen to 1.06 V?If needed, use the following equation: \Delta G° = -nFE°, E° , E = E° - , moles e<sup>-</sup> = (If needed, refer to Table 17-1in the text)</strong> A) [Cu<sup>2+</sup>]= 8.5 x 10<sup>-2</sup> M; [Zn<sup>2+</sup>]=1.91 M B) [Cu<sup>2+</sup>]= 0.94 M; [Zn<sup>2+</sup>]= 1.06 M C) [Cu<sup>2+</sup>]= 1.91 M; [Zn<sup>2+</sup>]=8.5 x 10<sup>-2</sup> M D) [Cu<sup>2+</sup>]= 0.50 M; [Zn<sup>2+</sup>]=1.50 M E) [Cu<sup>2+</sup>]= 0.90 M; [Zn<sup>2+</sup>]=1.10 M](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d433_2f97_827e_430b5f32088f_TB9687_11.jpg) , E = E° -
, E = E° - ![<strong>Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu<sup>2+</sup><sup> </sup>(aq) \rarr Zn<sup>2+</sup><sup> </sup>(aq) + Cu (s) \quad E° = 1.10 VIf the cell is initially at standard conditions ([Cu<sup>2+</sup>] = [Zn<sup>2+</sup>] = 1.00 M), what are the concentrations of Cu<sup>2+</sup><sup> </sup>and Zn<sup>2+</sup> when the cell potential has fallen to 1.06 V?If needed, use the following equation: \Delta G° = -nFE°, E° , E = E° - , moles e<sup>-</sup> = (If needed, refer to Table 17-1in the text)</strong> A) [Cu<sup>2+</sup>]= 8.5 x 10<sup>-2</sup> M; [Zn<sup>2+</sup>]=1.91 M B) [Cu<sup>2+</sup>]= 0.94 M; [Zn<sup>2+</sup>]= 1.06 M C) [Cu<sup>2+</sup>]= 1.91 M; [Zn<sup>2+</sup>]=8.5 x 10<sup>-2</sup> M D) [Cu<sup>2+</sup>]= 0.50 M; [Zn<sup>2+</sup>]=1.50 M E) [Cu<sup>2+</sup>]= 0.90 M; [Zn<sup>2+</sup>]=1.10 M](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d433_2f98_827e_cfe7754dd8fc_TB9687_11.jpg) , moles e- =
, moles e- = ![<strong>Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu<sup>2+</sup><sup> </sup>(aq) \rarr Zn<sup>2+</sup><sup> </sup>(aq) + Cu (s) \quad E° = 1.10 VIf the cell is initially at standard conditions ([Cu<sup>2+</sup>] = [Zn<sup>2+</sup>] = 1.00 M), what are the concentrations of Cu<sup>2+</sup><sup> </sup>and Zn<sup>2+</sup> when the cell potential has fallen to 1.06 V?If needed, use the following equation: \Delta G° = -nFE°, E° , E = E° - , moles e<sup>-</sup> = (If needed, refer to Table 17-1in the text)</strong> A) [Cu<sup>2+</sup>]= 8.5 x 10<sup>-2</sup> M; [Zn<sup>2+</sup>]=1.91 M B) [Cu<sup>2+</sup>]= 0.94 M; [Zn<sup>2+</sup>]= 1.06 M C) [Cu<sup>2+</sup>]= 1.91 M; [Zn<sup>2+</sup>]=8.5 x 10<sup>-2</sup> M D) [Cu<sup>2+</sup>]= 0.50 M; [Zn<sup>2+</sup>]=1.50 M E) [Cu<sup>2+</sup>]= 0.90 M; [Zn<sup>2+</sup>]=1.10 M](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d433_2f99_827e_3f1d17c3c852_TB9687_11.jpg) (If needed, refer to Table 17-1in the text)
(If needed, refer to Table 17-1in the text)A) [Cu2+]= 8.5 x 10-2 M; [Zn2+]=1.91 M
B) [Cu2+]= 0.94 M; [Zn2+]= 1.06 M
C) [Cu2+]= 1.91 M; [Zn2+]=8.5 x 10-2 M
D) [Cu2+]= 0.50 M; [Zn2+]=1.50 M
E) [Cu2+]= 0.90 M; [Zn2+]=1.10 M

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
28
What is the correct description, in line notation, for an electrochemical cell comprised of only Ag wire, AgNO3 electrolyte solution, and a salt bridge having G = -2 kJ?If needed, use the following equation: G° = -nFE°, E° 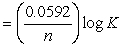 , E = E?
, E = E?
-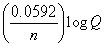 , moles e- =
, moles e- = 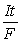 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
A) Ag(s) Ag+( aq, 1.00 M) Ag+(aq, 2.25 M) Ag(s)
B) Ag(s) Ag+( aq, 1.00 M) Ag+(aq, 1.00 M) Ag(s)
C) Ag(s) Ag+( aq, 2.25 M) Ag+(aq, 1.00 M) Ag(s)
D) Ag(s) Ag+( aq, 0.445 M) Ag+(aq, 1.00 M) Ag(s)
E) Ag(s) Ag+( aq, 1.00 M), Ag+(aq, 2.25 M) Ag(s)
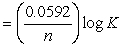 , E = E?
, E = E? -
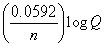 , moles e- =
, moles e- = 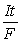 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )A) Ag(s) Ag+( aq, 1.00 M) Ag+(aq, 2.25 M) Ag(s)
B) Ag(s) Ag+( aq, 1.00 M) Ag+(aq, 1.00 M) Ag(s)
C) Ag(s) Ag+( aq, 2.25 M) Ag+(aq, 1.00 M) Ag(s)
D) Ag(s) Ag+( aq, 0.445 M) Ag+(aq, 1.00 M) Ag(s)
E) Ag(s) Ag+( aq, 1.00 M), Ag+(aq, 2.25 M) Ag(s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
29
For the working galvanic cell shown at standard conditions, how would you increase the cell potential?(If needed, refer to Table 17-1 in the text ) 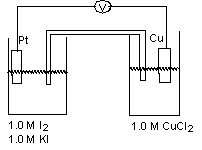
A) Make the Pt electrode larger.
B) Make the Copper electrode larger.
C) Increase the concentration of KI.
D) Increase the concentration of I2.
E) Make the Cu electrode smaller.

A) Make the Pt electrode larger.
B) Make the Copper electrode larger.
C) Increase the concentration of KI.
D) Increase the concentration of I2.
E) Make the Cu electrode smaller.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
30
For the following galvanic cell what will be its potential when the reaction reaches equilibrium?(If needed, refer to Table 17-1in the text ) 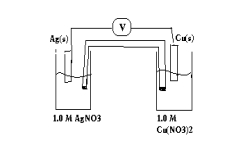
A) 0.0 V
B) 0.458 V
C) 1.142 V
D) 0.272 V
E) 1.26 V

A) 0.0 V
B) 0.458 V
C) 1.142 V
D) 0.272 V
E) 1.26 V

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
31
Ships, storage tanks, and other large metal items may be protected from corrosion by(If needed, refer to Table 17-1in the text )
A) a sacrificial cathode.
B) reduction of K+ to K.
C) a sacrificial anode.
D) coating with potassium metal.
E) there is no way to protect metals from corrosion.
A) a sacrificial cathode.
B) reduction of K+ to K.
C) a sacrificial anode.
D) coating with potassium metal.
E) there is no way to protect metals from corrosion.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
32
What are the possible oxidation states of corroded iron?(If needed, refer to Table 17-1 in the text)
A) 2
B) 0
C) 3
D) 2 and 3
E) 0, 2 and 3
A) 2
B) 0
C) 3
D) 2 and 3
E) 0, 2 and 3

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
33
How is aluminium protected from oxidation?(If needed, refer to Table 17-1in the text)
A) formation of an oxide layer
B) attaching it to a block of zinc
C) coating it with iron
D) Aluminium is not protected from oxidation, since Eo for Al is -1.662 V it will always corrode.
E) Provide a protective paint on the surface of the aluminium metal.
A) formation of an oxide layer
B) attaching it to a block of zinc
C) coating it with iron
D) Aluminium is not protected from oxidation, since Eo for Al is -1.662 V it will always corrode.
E) Provide a protective paint on the surface of the aluminium metal.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following combinations would provide the largest potential for a battery?(If needed, refer to Table 17-1in the text)
A) Br2 and Fe
B) Br-1 and Fe+2
C) Al and Cu+2
D) Al+3 and Cu+2
E) Al and Br-
A) Br2 and Fe
B) Br-1 and Fe+2
C) Al and Cu+2
D) Al+3 and Cu+2
E) Al and Br-

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
35
You have an abundant supply of NaCl salt from which you would like to prepare pure metallic sodium.
A) As the reduction potential of the aqueous Na+/Na couple is -1.662 V, this process is accomplished spontaneously in aqueous solution.
B) As the reduction potential of the aqueous Na+/Na couple is -1.662 V, this process is accomplished spontaneously from molten NaCl.
C) You prepare an aqueous solution of NaCl, apply a voltage of 1.662 V and collect metallic Na.
D) Na is produced by electrolysis of molten liquid NaCl at elevated temperature (NaCl mp. is 800oC).
E) Na is produced by electrolysis of solid NaCl.
A) As the reduction potential of the aqueous Na+/Na couple is -1.662 V, this process is accomplished spontaneously in aqueous solution.
B) As the reduction potential of the aqueous Na+/Na couple is -1.662 V, this process is accomplished spontaneously from molten NaCl.
C) You prepare an aqueous solution of NaCl, apply a voltage of 1.662 V and collect metallic Na.
D) Na is produced by electrolysis of molten liquid NaCl at elevated temperature (NaCl mp. is 800oC).
E) Na is produced by electrolysis of solid NaCl.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
36
Assign oxidation numbers to all the elements in HCO2H.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
37
Assign oxidation numbers to all the elements in NO2-.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
38
Assign oxidation numbers to all the elements in titanium nitride, Ti3N4.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
39
Assign oxidation numbers to all the elements in sodium bicarbonate, NaHCO3.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
40
Balance the following half reaction under neutral conditions:HSO3- SO42-

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
41
Balance the following half reaction under acidic conditions:I2O5 I2

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
42
Balance the following half reaction under basic conditions:
MnO4- MnO2(s)
MnO4- MnO2(s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
43
Balance the following half reaction under basic conditions:NO3- NO2-

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
44
Balance the following half reaction under acidic conditions:OCl- Cl-

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
45
Use the half-reaction method to balance the following redox reaction:
Cl2 Cl- + ClO- (basic solution)
Cl2 Cl- + ClO- (basic solution)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
46
Use the half-reaction method to balance the following redox reaction:
I2 + S2O32- I - + S4O62- (basic solution)
I2 + S2O32- I - + S4O62- (basic solution)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
47
If the coefficient of I- is 1, determine the number of electrons transferred:
OCl- + I- Cl- + IO-
OCl- + I- Cl- + IO-

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the standard free energy change for the following redox reaction: ( G = 77.11 (Ag+) and 65.49 kj/mol
(Cu+2)2 Ag+ (aq) + Cu (s) 2 Ag (s) + Cu2+ (aq)
(Cu+2)2 Ag+ (aq) + Cu (s) 2 Ag (s) + Cu2+ (aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
49
Draw three molecular pictures illustrating direct electron transfer in the reaction of silver (I) ions with copper metal.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
50
Draw a figure illustrating how a cell would be arranged for the redox reaction of copper with silver ion but using indirect electron transfer and a salt bridge with KNO3 solution. Indicate the direction of electron flow in the wire and the movement of ions in the salt bridge.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the redox process:
Cd(OH)2 + Ni(OH)2 + 2 OH- NiO2 + Cd + H2OWrite the equation for the spontaneous process and determine the free energy change for the spontaneous process.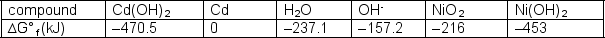
Cd(OH)2 + Ni(OH)2 + 2 OH- NiO2 + Cd + H2OWrite the equation for the spontaneous process and determine the free energy change for the spontaneous process.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
52
For the galvanic cell shown in the diagram, identify the anode and mark which direction the cations are moving in the salt bridge. 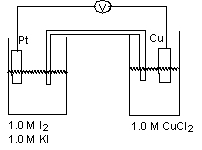


Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
53
Calculate the standard potential of voltaic cells that combine the following half reactions:Pb to PbSO4 and PbO2 to PbSO4 (acid solution)(If needed, refer to Table 17-1.in the text )

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
54
Calculate the standard potential of the redox reaction:
2 Na + S Na2S (E° for S + 2e- = S2- = -0.508 V(If needed, refer to Table 17-1. in the text)
2 Na + S Na2S (E° for S + 2e- = S2- = -0.508 V(If needed, refer to Table 17-1. in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the standard potential of the aluminium air battery in which the active materials Al(s) andO2, and the electrolyte is aqueous KOH.(If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
56
Balance the reaction and calculate the standard potential for:
Zr + H2O ZrO2 + H2Given: ZrO2 + 4e- + 4 H3O+ Zr + 6 H2O (E°=-1.43 V)(If needed, refer to Table 17-1 in the text)
Zr + H2O ZrO2 + H2Given: ZrO2 + 4e- + 4 H3O+ Zr + 6 H2O (E°=-1.43 V)(If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
57
An electrochemical cell is constructed that contains Cr3+(aq) and Cr metal as the electrode in one compartment and Cu2+(aq) and copper metal in the other compartment. Calculate the expected standard potential upon appropriately connecting the cell and describe the direction of electron and cation flow.(If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the species listed is the strongest oxidizing agent?  (If needed, refer to Table 17-1 in the text)
(If needed, refer to Table 17-1 in the text)
 (If needed, refer to Table 17-1 in the text)
(If needed, refer to Table 17-1 in the text)
Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the species listed is the strongest reducing agent? 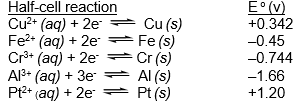 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate the standard free energy change for the redox reaction between silver ion and copper to give copper (II) and silver metal.(If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
61
Calculate the standard free energy changes for the following redox reaction:
2 Ag+(aq) + Sn2+(aq) 2 Ag(s) + Sn4+(aq) [E°(Sn4+, 2+) = 0.151 V](If needed, refer to Table 17-1 in the text)
2 Ag+(aq) + Sn2+(aq) 2 Ag(s) + Sn4+(aq) [E°(Sn4+, 2+) = 0.151 V](If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
62
Calculate the equilibrium constant for the following redox reaction:
Fe3+ (aq) + Cu+ (aq) Fe2+ (aq) + Cu2+ (aq)[E°(Fe3+, 2+) = 0.771 V] [E°(Cu2+,1+) = 0.153 V](If needed, refer to Table 17-1 in the text)
Fe3+ (aq) + Cu+ (aq) Fe2+ (aq) + Cu2+ (aq)[E°(Fe3+, 2+) = 0.771 V] [E°(Cu2+,1+) = 0.153 V](If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
63
Calculate the equilibrium constants for the following redox reaction:
2 Cu2+ (aq) + Sn2+ (aq) 2 Cu+ (aq) + Sn4+ (aq)[E°(Sn4+, 2+) = 0.151 V] [E°(Cu2+, 1+) = 0.153 V](If needed, refer to Table 17-1 in the text)
2 Cu2+ (aq) + Sn2+ (aq) 2 Cu+ (aq) + Sn4+ (aq)[E°(Sn4+, 2+) = 0.151 V] [E°(Cu2+, 1+) = 0.153 V](If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the Daniell cell for which the cell reaction and standard potential are:Zn(s) + Cu2+ (aq) Zn2+ (aq) + Cu (s) E° = 1.10 VIf the cell is initially at standard conditions ([Cu2+] = [Zn2+] = 1.00 M) and assuming it contains 1 L of electrolyte, determine the mass of Zn(s) lost when the cell potential falls to 1.06 V?(If needed, refer to Table 17-1in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
65
Consider an electrochemical cell of the type shown in the figure where the redox half-reaction in both compartments has the identical standard potentials: 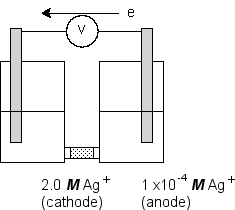 Use the Nernst equation to calculate the potential developed by this cell.(If needed, refer to Table 17-1 in the text)
Use the Nernst equation to calculate the potential developed by this cell.(If needed, refer to Table 17-1 in the text)
 Use the Nernst equation to calculate the potential developed by this cell.(If needed, refer to Table 17-1 in the text)
Use the Nernst equation to calculate the potential developed by this cell.(If needed, refer to Table 17-1 in the text)
Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
66
Aluminium is used in a battery in which the following reaction occurs:
4 Al (s) + 3 O2 (g) + 4 OH- (aq) + 6 H2O 4 Al(OH) (-,4) (aq)If the battery must supply a current of 78 A for 4.0 hours, what mass of Al (ing) must be contained in the battery?(If needed, refer to Table 17-1 in the text)
4 Al (s) + 3 O2 (g) + 4 OH- (aq) + 6 H2O 4 Al(OH) (-,4) (aq)If the battery must supply a current of 78 A for 4.0 hours, what mass of Al (ing) must be contained in the battery?(If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
67
An electrochemical cell is made by immersing a piece of Cd metal into a solution of 0.100 M CdSO4 and a Zn electrode into a solution of 1.00 M ZnSO4 and placing a salt bridge to allow ion flow between the two solutions.a) What voltage will be produced by the cell and b) what metal is the anode? (Cd2+ + 2e- Cd; E° = -0.402 V)(If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
68
The lead-acid battery used in automobiles utilizes the following redox reaction:
PbO2(s) + Pb (s) + 2 HSO4- (aq) + 2 H3O+ (aq) 2 PbSO4(s) + 4 H2O (l) E°= 2.04 VWhat mass of H2 being oxidized by O2 under standard acid conditions would be required to give the same amount of electrons as one mole of lead oxide?(If needed, refer to Table 17-1 in the text)
PbO2(s) + Pb (s) + 2 HSO4- (aq) + 2 H3O+ (aq) 2 PbSO4(s) + 4 H2O (l) E°= 2.04 VWhat mass of H2 being oxidized by O2 under standard acid conditions would be required to give the same amount of electrons as one mole of lead oxide?(If needed, refer to Table 17-1 in the text)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
69
Consider an automobile which is powered by a perfectly efficient fuel cell that consumes hydrogen and oxygen in the following redox reaction:
2H2(g) + O2(g) 2 H2O (l)If the electric system requires a current of 500 amperes, how many g of H2 are consumed per hour?(If needed, refer to Table 17-1 in the text )
2H2(g) + O2(g) 2 H2O (l)If the electric system requires a current of 500 amperes, how many g of H2 are consumed per hour?(If needed, refer to Table 17-1 in the text )

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
70
For the working galvanic cell shown at standard conditions, determine the balanced reaction and direction of electron flow through the wire. 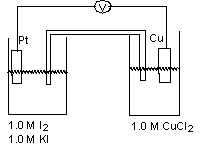 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
71
The same charge of 1.07 x 104 C is passed through three solutions: one each of Au3+, Cu+ and Pb2+ with strips of the metals as cathodes. In which cell will the greatest mass of metal be reduced and what is the mass of that metal?

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
72
For a brine electrolysis cell (see redox reaction below) operating at 60,000 amps, how many kg of NaOH and Cl2 would be produced in 24.0 hours?
2 NaCl (aq) + 2 H2O 2 NaOH (aq) + Cl2(g) + H2 (g)
2 NaCl (aq) + 2 H2O 2 NaOH (aq) + Cl2(g) + H2 (g)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
73
An electrolytic cell driving the following redox reaction has a current of 4.02 amps passed through it for 2.32 hours. How much Ag will be dissolved and how much Cu will be deposited?2 Ag (s) + Cu+2 (aq) 2 Ag+ (aq) + Cu (s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
74
Determine what causes the following electrolytic cell (which includes 50 g of metallic Ag and 1 L of 0.15 M Cu(NO3)2 ) to cease operation and determine how long the cell can sustain a current of 5 amps.
2 Ag (s) + Cu+2 (aq) 2 Ag+ (aq) + Cu (s)
2 Ag (s) + Cu+2 (aq) 2 Ag+ (aq) + Cu (s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
75
At an engine block rebuilding factory you are in charge of replating Mn on the interiors of engine blocks. Based on the surface area and thickness needed, you determine that you need 35g of Mn to plate out by performing electrolysis on the engine block. Your plating solution is 3 M Mn(NO3)2. How long do you need to perform electrolysis if your machine performs at 220 Amps?

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
76
You determine that for proper protection of an engine part you need to put a coating of 3.0g of Cr(s) on your part. How long do you need to perform electrolysis on your engine part if your current is 30.0 Amps, and your Cr is in the form of Cr(NO3)3(aq)?

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck



