Deck 8: Nuclear Physics and Atomic Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/9
Play
Full screen (f)
Deck 8: Nuclear Physics and Atomic Physics
1
The following masses are known:
u
u
u
The binding energy of , in MeV, is closest to:
A) 1700
B) 1900
C) 2100
D) 2300
E) 2500
u
u
u
The binding energy of , in MeV, is closest to:
A) 1700
B) 1900
C) 2100
D) 2300
E) 2500
1700
2
Today, uranium contains 0.72% 235U (half-life = 0.70 billion years) and 99.28% 238U (half-life = 4.5 billion years). At a time 1.9 billion years ago, what was the fraction of 235U in uranium?
A) 3.53%
B) 4.72%
C) 4.90%
D) 6.75%
A) 3.53%
B) 4.72%
C) 4.90%
D) 6.75%
3.53%
3
Neodymium 144Nd is a nuclide that undergoes alpha decay. The nuclide that is the product of the decay is:
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)
4
Scandium 44Sc decays by emitting a positron. The nuclide that is the product of the decay is:
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
5
The stability of with respect to alpha, beta-plus, and beta-minus decay is to be determined. The following atomic masses in amu are known:
4.002603
7.016928
11.009305
11.011433
11.026742
The nuclide is
A) not subject to alpha, beta-plus, or beta-minus decay.
B) subject to alpha decay only.
C) subject to beta-plus decay only.
D) subject to beta-minus decay only.
E) subject to beta-plus or beta-minus decay, but not to alpha decay.
4.002603
7.016928
11.009305
11.011433
11.026742
The nuclide is
A) not subject to alpha, beta-plus, or beta-minus decay.
B) subject to alpha decay only.
C) subject to beta-plus decay only.
D) subject to beta-minus decay only.
E) subject to beta-plus or beta-minus decay, but not to alpha decay.

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
6
The stability of with respect to alpha, beta-plus, and beta-minus decay is to be determined. The following atomic masses in amu are known:
4.002603
31.973907
35.967081
35.968307
35.967546
The
nuclide is
A) not subject to alpha, beta-plus, or beta-minus decay.
B) subject to alpha decay only.
C) subject to beta-plus decay only.
D) subject to beta-minus decay only.
E) subject to beta-plus or beta-minus decay, but not to alpha decay.
4.002603
31.973907
35.967081
35.968307
35.967546
The
nuclide is
A) not subject to alpha, beta-plus, or beta-minus decay.
B) subject to alpha decay only.
C) subject to beta-plus decay only.
D) subject to beta-minus decay only.
E) subject to beta-plus or beta-minus decay, but not to alpha decay.

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
7
If Lz = 0 for the hydrogen atom, the electron is not orbiting because it has no angular momentum.

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the statements below is true?
A) For a particular shell, an electron in a hydrogen atom with a higher angular momentum is more likely to be found in a smaller range of distances from the proton.
B) For a particular shell, an electron in a hydrogen atom with a higher angular momentum is more likely to be found in a larger range of distances from the proton.
C) For a particular shell, an electron in a hydrogen atom with a higher angular momentum will have a higher mechanical energy.
D) For a particular shell, an electron in a hydrogen atom with a higher angular momentum is more likely to be found farther from the proton.
A) For a particular shell, an electron in a hydrogen atom with a higher angular momentum is more likely to be found in a smaller range of distances from the proton.
B) For a particular shell, an electron in a hydrogen atom with a higher angular momentum is more likely to be found in a larger range of distances from the proton.
C) For a particular shell, an electron in a hydrogen atom with a higher angular momentum will have a higher mechanical energy.
D) For a particular shell, an electron in a hydrogen atom with a higher angular momentum is more likely to be found farther from the proton.

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
9
What is the greatest magnitude of the orbital angular momentum L that you can find in a state with n = 6?
A) 5.48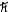
B) 5.92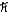
C) 6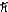
D) 6.48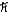
A) 5.48
B) 5.92
C) 6
D) 6.48

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck



