Deck 12: Applied General Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/139
Play
Full screen (f)
Deck 12: Applied General Chemistry
1
what is this symble for:
-H
-H
Hydrogen
2
what is this symble for:
-Li
-Li
Lithium
3
what is this symble for:
-Be
-Be
Beryllium
4
what is this symble for:
-Na
-Na

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
5
what is this symble for:
-Mg
-Mg

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
6
what is this symble for:
-K
-K

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
7
what is this symble for:
-Ca
-Ca

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
8
what is this symble for:
-Sr
-Sr

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
9
what is this symble for:
-Ra
-Ra

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
10
what is this symble for:
-Ti
-Ti

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
11
what is this symble for:
-Zr
-Zr

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
12
what is this symble for:
-Cr
-Cr

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
13
what is this symble for:
-Mo
-Mo

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
14
what is this symble for:
-W
-W

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
15
what is this symble for:
-Mn
-Mn

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
16
what is this symble for:
-Fe
-Fe

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
17
what is this symble for:
-Co
-Co

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
18
what is this symble for:
-Ir
-Ir

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
19
what is this symble for:
-Ni
-Ni

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
20
what is this symble for:
-Pd
-Pd

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
21
what is the symbole for each term:
-Platinum
-Platinum

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
22
what is the symbole for each term:
-Copper
-Copper

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
23
what is the symbole for each term:
-Silver
-Silver

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
24
what is the symbole for each term:
-Gold
-Gold

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
25
what is the symbole for each term:
-Zinc
-Zinc

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
26
what is the symbole for each term:
-Cadium
-Cadium

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
27
what is the symbole for each term:
-Mercury
-Mercury

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
28
what is the symbole for each term:
-Boron
-Boron

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
29
what is the symbole for each term:
-Aluminum
-Aluminum

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
30
what is the symbole for each term:
-Gallium
-Gallium

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
31
what is the symbole for each term:
-Carbon
-Carbon

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
32
what is the symbole for each term:
-Silicon
-Silicon

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
33
what is the symbole for each term:
-Germanium
-Germanium

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
34
what is the symbole for each term:
-Tin
-Tin

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
35
what is the symbole for each term:
-Lead
-Lead

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
36
what is the symbole for each term:
-Nitrogen
-Nitrogen

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
37
what is the symbole for each term:
-Phosphorus
-Phosphorus

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
38
what is the symbole for each term:
-Arsenic
-Arsenic

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
39
what is the symbole for each term:
-Antimony
-Antimony

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
40
what is the symbole for each term:
-Bismuth
-Bismuth

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
41
what is the symbole for each term:
-Oxygen
-Oxygen

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
42
what is this symble for:
-S
-S

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
43
what is this symble for:
-Se
-Se

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
44
what is this symble for:
-F
-F

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
45
what is this symble for:
-Cl
-Cl

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
46
what is this symble for:
-Br
-Br

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
47
what is the symbole for each term:
-Iodine
-Iodine

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
48
what is the symbole for each term:
-Helium
-Helium

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
49
what is the symbole for each term:
-Neon
-Neon

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
50
what is this symble for:
-Ar
-Ar

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
51
what is the symbole for each term:
-Krypton
-Krypton

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
52
what is this symble for:
-Xe
-Xe

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
53
what is the symbole for each term:
-Radon
-Radon

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
54
what is this symble for:
-Pu
-Pu

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
55
what is the symbole for each term:
-Uranium
-Uranium

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
56
Chemistry is defined as:
A) the study of the changes that occur when chemical elements combine to form other substances.
B) the study of life
C) the study of the structure or characteristics of elements
D) a & c
A) the study of the changes that occur when chemical elements combine to form other substances.
B) the study of life
C) the study of the structure or characteristics of elements
D) a & c

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
57
2H3PO4 --» H2O + H4P2O7. Is this chemical equation balanced? Show work!
A) balanced
B) not balanced
A) balanced
B) not balanced

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
58
Uses a porous solid to remove gases or liquids from a mixture.
A) absorption
B) adsorption
C) porosity
D) extraction
A) absorption
B) adsorption
C) porosity
D) extraction

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
59
_______________ removes one or more components from a gas mixture by exposing it to a gas or liquid.
A) adsorption
B) desorb
C) absorption
D) distillation
A) adsorption
B) desorb
C) absorption
D) distillation

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
60
The __________ can be found in the outer-most shell of an atom.
A) electron
B) neutron
C) valence electron
D) proton
A) electron
B) neutron
C) valence electron
D) proton

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
61
A 2500 pound barrel has a 37% catalyst solution. What is the weight of the catalyst?
A) 67.6 lbs
B) 925 lbs
C) 92500 lbs
D) 1143 lbs
A) 67.6 lbs
B) 925 lbs
C) 92500 lbs
D) 1143 lbs

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
62
Given the chemical equation: H3PO4 + 3 NaOH→Na3PO4 + 3H2O
If you are told to increase H3PO4 from 98 GPM to 294 GPM, what will the settings be for the other reactant and products? Show your work
.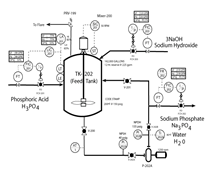
If you are told to increase H3PO4 from 98 GPM to 294 GPM, what will the settings be for the other reactant and products? Show your work
.


Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
63
A(n) _________ is the smallest particle of a(n) __________ that still retains the characteristics of a(n) __________ .
A) element, atom solution
B) atom, element, element
C) atom, element, compound
D) element, atom, atom
A) element, atom solution
B) atom, element, element
C) atom, element, compound
D) element, atom, atom

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
64
An acid is:
A) a bitter tasting chemical compound that has a pH value below 7.0, changes blue litmus to red, yields hydrogen ions in water.
B) a bitter tasting chemical compound that has a soapy feel and a pH value above 7.0, changes red litmus blue and yields hydroxyl ions.
C) a bitter tasting chemical compound that has a soapy feel and a pH value between 0 and 14, changes green litmus blue and yields hydroxyl and hydrogen ions.
D) a sweet tasting chemical solution that has a fluctuating pH value, changes orange litmus green, yields sodium ions above the 50 percentile range.
A) a bitter tasting chemical compound that has a pH value below 7.0, changes blue litmus to red, yields hydrogen ions in water.
B) a bitter tasting chemical compound that has a soapy feel and a pH value above 7.0, changes red litmus blue and yields hydroxyl ions.
C) a bitter tasting chemical compound that has a soapy feel and a pH value between 0 and 14, changes green litmus blue and yields hydroxyl and hydrogen ions.
D) a sweet tasting chemical solution that has a fluctuating pH value, changes orange litmus green, yields sodium ions above the 50 percentile range.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
65
2Na2O + 2HOCl --» 2NaOCl + H2O. Is this chemical equation balanced? List reactant elements. List the product elements.
A) balanced
B) not balanced
A) balanced
B) not balanced

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
66
The major difference between the two most common forms of chemical bonding are:
A) neutral vs electrically charged
B) endothermic vs. exothermic
C) reactive vs. equilibrium
D) all of the above
A) neutral vs electrically charged
B) endothermic vs. exothermic
C) reactive vs. equilibrium
D) all of the above

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
67
The chemical name for Gold:
A) Ag
B) Au
C) G
D) Gd
A) Ag
B) Au
C) G
D) Gd

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
68
The chemical name for Antimony:
A) Sb
B) A
C) An
D) Sn
A) Sb
B) A
C) An
D) Sn

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
69
All of the following are states of matter except:
A) plasma and gas
B) solid and liquid
C) liquid and energy
D) plasma and solid
A) plasma and gas
B) solid and liquid
C) liquid and energy
D) plasma and solid

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
70
The chemical name for Sulfur:
A) Sr
B) S
C) Ag
D) Cl
A) Sr
B) S
C) Ag
D) Cl

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
71
The ______________________ provides information about all known elements.
A) chemical element chart
B) periodic table
C) element table
D) a & b
A) chemical element chart
B) periodic table
C) element table
D) a & b

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
72
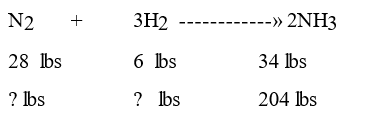
What are the new settings for nitrogen and hydrogen?
A) 420 nitrogen, 136 hydrogen
B) 410 nitrogen, 146 hydrogen
C) 395 nitrogen, 161 hydrogen
D) 168 nitrogen, 36 hydrogen

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
73
The chemical name for Magnesium:
A) Mn
B) Mg
C) Mo
D) Unh
A) Mn
B) Mg
C) Mo
D) Unh

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
74
The chemical name for Silicon:
A) Si
B) S
C) Sb
D) Cs
A) Si
B) S
C) Sb
D) Cs

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
75
The atomic number is determined by:
A) number of electrons
B) number of protons
C) number of AMU's
D) number of neutrons
A) number of electrons
B) number of protons
C) number of AMU's
D) number of neutrons

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
76
Elements _____________ be broken down or changed by chemical or physical means.
A) can
B) can not
C) should not
D) should
A) can
B) can not
C) should not
D) should

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
77
The two most common forms of chemical bonding are:
A) covalent and ionic
B) covalent and numeric
C) ionic and electromagnetic
D) covalent and memonic
A) covalent and ionic
B) covalent and numeric
C) ionic and electromagnetic
D) covalent and memonic

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
78
The chemical name for Tungsten:
A) W
B) T
C) Tn
D) As
A) W
B) T
C) Tn
D) As

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
79
An exothermic reaction:
A) requires heat
B) requires energy
C) gives off heat
D) destroys matter
A) requires heat
B) requires energy
C) gives off heat
D) destroys matter

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
80
How does heat and pressure effect a chemical reaction?
A) enhances
B) slows down
C) no effect
D) a & b
A) enhances
B) slows down
C) no effect
D) a & b

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck



