Deck 12: Oxidation-Reduction Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/81
Play
Full screen (f)
Deck 12: Oxidation-Reduction Reactions
1

Determining Oxidation Numbers
-What is the oxidation number of the carbon in bold print in the following structure?
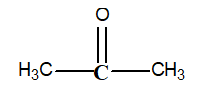
A) -1
B) +2
C) -3
D) +4
E) none of these

Determining Oxidation Numbers
-What is the oxidation number of the carbon in bold print in the following structure?

A) -1
B) +2
C) -3
D) +4
E) none of these
+2
2

Determining Oxidation Numbers
-Determine the oxidation number of the bold face carbon atom in pyruvic acid:
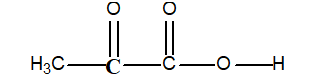
A)-2
B) +2
C) +3
D) +4
E) none of these

Determining Oxidation Numbers
-Determine the oxidation number of the bold face carbon atom in pyruvic acid:

A)-2
B) +2
C) +3
D) +4
E) none of these
+2
3
? 
Determining Oxidation Numbers
-Determine the oxidation number for bromine in HBrO4.
A) +6
B) +7
C) +8
D) -6
E) -7

Determining Oxidation Numbers
-Determine the oxidation number for bromine in HBrO4.
A) +6
B) +7
C) +8
D) -6
E) -7
+7
4
? 
Determining Oxidation Numbers
-Determine the oxidation state of the carbon atom in bold print:
CH3CH2F
A) 0
B) +1
C) +2
D) +3
E)-1

Determining Oxidation Numbers
-Determine the oxidation state of the carbon atom in bold print:
CH3CH2F
A) 0
B) +1
C) +2
D) +3
E)-1

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
5
? 
Determining Oxidation Numbers
-Determine the oxidation number of arsenic in the anion, HAsO42 - .
A) +5
B) +6
C) +7
D) +8
E) none of these

Determining Oxidation Numbers
-Determine the oxidation number of arsenic in the anion, HAsO42 - .
A) +5
B) +6
C) +7
D) +8
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
6

Determining Oxidation Numbers
-Determine the oxidation state of the carbon atom shown in bold in the following compound:
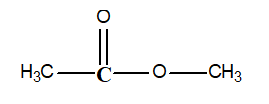
A) -1
B) 0
C) +1
D) +4
E) none of these

Determining Oxidation Numbers
-Determine the oxidation state of the carbon atom shown in bold in the following compound:

A) -1
B) 0
C) +1
D) +4
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
7
The carbon in bold in the ethanol has an oxidation number of __ while the carbon in bold in acetic acid has an oxidation number of ___.
A) -1, +3
B) +1, +2
C) -2, +3
D) +2, -3
E) none of the above
A) -1, +3
B) +1, +2
C) -2, +3
D) +2, -3
E) none of the above

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about this reaction is correct?
A) H+ is the oxidizing agent and dichromate is the reducing agent.
B) Dichromate is oxidized and ethanol is reduced.
C) Ethanol is the reducing agent and dichromate is the oxidizing agent.
D) This is not an oxidation-reduction reaction.
E) None of the above statements is correct.
A) H+ is the oxidizing agent and dichromate is the reducing agent.
B) Dichromate is oxidized and ethanol is reduced.
C) Ethanol is the reducing agent and dichromate is the oxidizing agent.
D) This is not an oxidation-reduction reaction.
E) None of the above statements is correct.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
9
How many electrons are transferred in the following oxidation-reduction reaction?
3 Sn2+(aq) + Cr2O72-(aq) + 14 H+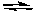 3 Sn4+(aq) + 2 Cr3+(aq) + 7 H2O(l)
3 Sn4+(aq) + 2 Cr3+(aq) + 7 H2O(l)
A) 2
B) 6
C) 12
D) 14
E) none of these
3 Sn2+(aq) + Cr2O72-(aq) + 14 H+
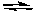 3 Sn4+(aq) + 2 Cr3+(aq) + 7 H2O(l)
3 Sn4+(aq) + 2 Cr3+(aq) + 7 H2O(l)A) 2
B) 6
C) 12
D) 14
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following isn't an example of an oxidation-reduction reaction?
A) Ca3P2(s) + 6 H2O(l) 3 Ca2+(aq) + 6 OH-(aq) + 2 PH3(g)
B) 2 PH3(g) + 4 O2(g) H3PO4(s)
C) P4(s) + 5 O2(g) P4O10(s)
D) 6 Ca(s) + P4(s) 2 Ca3P2(s)
E) All of the above are oxidation-reduction reactions.
A) Ca3P2(s) + 6 H2O(l) 3 Ca2+(aq) + 6 OH-(aq) + 2 PH3(g)
B) 2 PH3(g) + 4 O2(g) H3PO4(s)
C) P4(s) + 5 O2(g) P4O10(s)
D) 6 Ca(s) + P4(s) 2 Ca3P2(s)
E) All of the above are oxidation-reduction reactions.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following isn't an example of an oxidation-reduction reaction?
A) H2(g) + Cl2(g) 2 HCl(g)
B) Ag+(aq) + 2 NH3(g)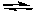 Ag(NH3)2+(aq)
Ag(NH3)2+(aq)
C) Hg2Cl2(s) + NH3(g) Hg(s) + HgNH2Cl(s)
D) 2 Mg(s) + O2(g) 2MgO(s)
E) Cl2(g) + 2 Br-(aq) 2 Cl-(aq) + Br2(l)
A) H2(g) + Cl2(g) 2 HCl(g)
B) Ag+(aq) + 2 NH3(g)
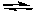 Ag(NH3)2+(aq)
Ag(NH3)2+(aq)C) Hg2Cl2(s) + NH3(g) Hg(s) + HgNH2Cl(s)
D) 2 Mg(s) + O2(g) 2MgO(s)
E) Cl2(g) + 2 Br-(aq) 2 Cl-(aq) + Br2(l)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
12
A voltaic cell is constructed with a Pt wire in an aqueous solution of Br2/Br - (1M) serving as the cathode and a silver wire in a 1M solution of Ag+ serving as the anode. The potential of the cell was measured to be +0.287 V. The standard reduction potential for Br2/Br - is 1.087 V.
(I) Write the half-reaction for the reduction taking place in the cell.
(II) Write the half-reaction for the oxidation taking place in the cell.
(III) Write the overall reaction for the oxidation-reduction reaction taking place in the cell.
(IV) Calculate the potential for the Ag/Ag+ half-reaction taking place in the cell.
(V) Determine the standard reduction potential, E°, for the half-reaction below.
Ag+(aq) + e- Ag(s)
(I) Write the half-reaction for the reduction taking place in the cell.
(II) Write the half-reaction for the oxidation taking place in the cell.
(III) Write the overall reaction for the oxidation-reduction reaction taking place in the cell.
(IV) Calculate the potential for the Ag/Ag+ half-reaction taking place in the cell.
(V) Determine the standard reduction potential, E°, for the half-reaction below.
Ag+(aq) + e- Ag(s)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
13
What will be the coefficients of Ce4+ and Cl - , respectively when the following reaction is balanced?
Ce4+(aq) + Cl - (aq) Cl2(aq) + Ce3+(aq)
A) 1,1
B) 1,2
C) 2.1
D) 2.2
E) 3,1
Ce4+(aq) + Cl - (aq) Cl2(aq) + Ce3+(aq)
A) 1,1
B) 1,2
C) 2.1
D) 2.2
E) 3,1

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
14
Use a table of standard reduction potentials to determine which of the following statements is true for the electrochemical cell diagrammed below. 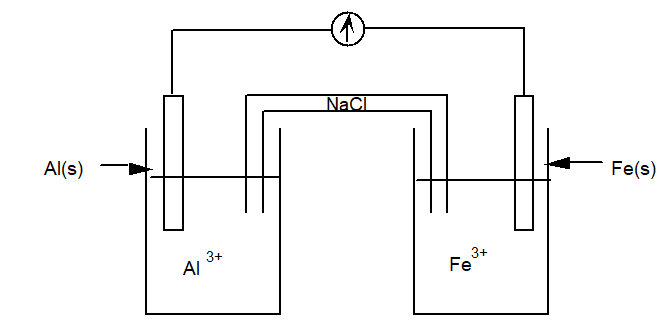
A) The Al is the cathode.
B) Electrons move from the Al electrode to the Fe electrode.
C) The mass of the Al electrode decreases.
D) Both (b) and (c) are true. .
E) Both (a) and (c) are true

A) The Al is the cathode.
B) Electrons move from the Al electrode to the Fe electrode.
C) The mass of the Al electrode decreases.
D) Both (b) and (c) are true. .
E) Both (a) and (c) are true

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
15
Use the table of electrode potentials to determine which of the following reactions isn't spontaneous under standard conditions.
A) Zn(s) + 2 H+(aq) Zn2+(aq) + H2(g)
B) 2 Ag(s) + Zn2+(aq) 2 Ag+(aq) + Zn(s)
C) Cl2(aq) + 2 Fe2+(aq) 2 Cl-(aq) + 2 Fe3+(aq)
D) 2 Al(s) + 3/2 O2(g) + 6 H+(aq) 2 Al3+(aq) + 3 H2O(l)
E) Mg(s) + Cl2(g) MgCl2(s)
A) Zn(s) + 2 H+(aq) Zn2+(aq) + H2(g)
B) 2 Ag(s) + Zn2+(aq) 2 Ag+(aq) + Zn(s)
C) Cl2(aq) + 2 Fe2+(aq) 2 Cl-(aq) + 2 Fe3+(aq)
D) 2 Al(s) + 3/2 O2(g) + 6 H+(aq) 2 Al3+(aq) + 3 H2O(l)
E) Mg(s) + Cl2(g) MgCl2(s)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the following generalized half-reactions.
Aox + e-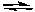 Ared
Ared
Box + e-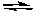 Bred
Bred
If E° for the half-reaction involving A is positive and if E° for the half-reaction involving B is negative, then at standard conditions:
A) Aox will reduce Box
B) Ared will reduce Box
C) Bred will reduce Aox
D) Bred will reduce Ared
E) no reaction will occur
Aox + e-
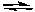 Ared
AredBox + e-
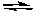 Bred
BredIf E° for the half-reaction involving A is positive and if E° for the half-reaction involving B is negative, then at standard conditions:
A) Aox will reduce Box
B) Ared will reduce Box
C) Bred will reduce Aox
D) Bred will reduce Ared
E) no reaction will occur

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
17
The potential of a cell at standard state conditions can be best be described as:
A) Eo
B) 0 volts
C) negative
D) more than one volt
E) None of the above
A) Eo
B) 0 volts
C) negative
D) more than one volt
E) None of the above

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
18
What is the potential for a voltaic cell produced from the following half-reactions, if all ions are present at 1.0 M concentrations?
Oxidation: Al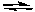 Al3+ + 3 e-
Al3+ + 3 e-
Reduction: Zn2+ + 2 e-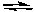 Zn
Zn
A) -3.59 V
B) -0.943 V
C) 0.943 V
D) 1.12 V
E) 3.59 V
Oxidation: Al
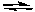 Al3+ + 3 e-
Al3+ + 3 e-Reduction: Zn2+ + 2 e-
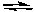 Zn
ZnA) -3.59 V
B) -0.943 V
C) 0.943 V
D) 1.12 V
E) 3.59 V

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
19
What is the standard cell potential for the following redox reaction?
2 Fe3+(aq) + 2 I-(aq) 2 Fe2+(aq) + I2(aq)
A) -1.30 V
B) -0.24 V
C) 0.24 V
D) 1.30 V
E) none of the above
2 Fe3+(aq) + 2 I-(aq) 2 Fe2+(aq) + I2(aq)
A) -1.30 V
B) -0.24 V
C) 0.24 V
D) 1.30 V
E) none of the above

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
20
What is the magnitude of the standard-state cell potential for the following redox reaction?
2 Al(s) + 3 Pb2+(aq) 2 Al3+(aq) + 3 Pb(s)
A) 1.58 V
B) 1.83 V
C) 3.03 V
D) 3.790 V
E) 4.866 V
2 Al(s) + 3 Pb2+(aq) 2 Al3+(aq) + 3 Pb(s)
A) 1.58 V
B) 1.83 V
C) 3.03 V
D) 3.790 V
E) 4.866 V

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
21
Oxidation-reduction reactions are often written with double arrows indicating that the reaction can go in either direction depending on the experimental conditions. For the following reaction identify the oxidizing agent on both sides of the chemical equation.
S2O82-(aq) + Zn(s)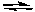 Zn2+(aq) + 2 SO42-(aq)
Zn2+(aq) + 2 SO42-(aq)
A) S2O82- and SO42-
B) Zn and SO42-
C) Zn and Zn2+
D) S2O82- and Zn2+
E) none of these
S2O82-(aq) + Zn(s)
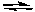 Zn2+(aq) + 2 SO42-(aq)
Zn2+(aq) + 2 SO42-(aq)A) S2O82- and SO42-
B) Zn and SO42-
C) Zn and Zn2+
D) S2O82- and Zn2+
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
22
Which statement correctly describes the following oxidation-reduction reaction?
5Cr3+(aq) + 3 MnO4 - (aq) + 8 H2O (l) 5 CrO42 - (aq) + 3 Mn2+(aq) + 16 H+(aq)
A) MnO4 - and H2O are both reduced.
B) Cr3+ is the reducing agent.
C) Cr3+ and H2O are both reducing agents.
D) MnO4 - is oxidized.
E) None of these are correct.
5Cr3+(aq) + 3 MnO4 - (aq) + 8 H2O (l) 5 CrO42 - (aq) + 3 Mn2+(aq) + 16 H+(aq)
A) MnO4 - and H2O are both reduced.
B) Cr3+ is the reducing agent.
C) Cr3+ and H2O are both reducing agents.
D) MnO4 - is oxidized.
E) None of these are correct.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is true about the reaction below?
2VO2+ (aq) + Br2(s) + 2 H2O(l) 2VO2+(aq) + 2 Br - (aq) + 4H+
A) Br2 is acting as a reducing agent.
B) VO2+ is acting as a weak reducing agent.
C) VO2+ is oxidized.
D) H2O is reduced.
E) H+ is acting as a weak reducing agent.
2VO2+ (aq) + Br2(s) + 2 H2O(l) 2VO2+(aq) + 2 Br - (aq) + 4H+
A) Br2 is acting as a reducing agent.
B) VO2+ is acting as a weak reducing agent.
C) VO2+ is oxidized.
D) H2O is reduced.
E) H+ is acting as a weak reducing agent.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
24
Identify each of the requested compounds for the following redox reaction.
2 MnO4 - (aq) + 16 H+(aq) + 10 Cl - (aq) 2 Mn2+(aq) + 8 H2O + 5 Cl2(g)
(I) oxidizing agent
(II) reducing agent
(III) conjugate oxidizing agent
(IV) conjugate reducing agent
2 MnO4 - (aq) + 16 H+(aq) + 10 Cl - (aq) 2 Mn2+(aq) + 8 H2O + 5 Cl2(g)
(I) oxidizing agent
(II) reducing agent
(III) conjugate oxidizing agent
(IV) conjugate reducing agent

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
25
Which statement correctly describes the following reaction?
O2(g) + 4 H+(aq) + 4 Cl - (aq) 2 H2O(l) + 2 Cl2(g)
A) O2 is a reducing agent.
B) H+ and Cl - are oxidized.
C) H+ is reduced.
D) H+ is a reducing agent.
E) Cl - is a reducing agent.
O2(g) + 4 H+(aq) + 4 Cl - (aq) 2 H2O(l) + 2 Cl2(g)
A) O2 is a reducing agent.
B) H+ and Cl - are oxidized.
C) H+ is reduced.
D) H+ is a reducing agent.
E) Cl - is a reducing agent.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
26
For the following reaction, identify the oxidizing agents in the forward and reverse directions, respectively..
P4(s) + HNO3(aq) H3PO4(aq) + NO(g)
A) P4 and H3PO4
B) P4 and NO
C) P4 and HNO3
D) HNO3 and H3PO4
E) HNO3 and NO
P4(s) + HNO3(aq) H3PO4(aq) + NO(g)
A) P4 and H3PO4
B) P4 and NO
C) P4 and HNO3
D) HNO3 and H3PO4
E) HNO3 and NO

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
27
For the reaction:
3 Sn2+(aq) + Cr2O72-(aq) + 14 H+(aq) 3 Sn4+(aq) + 2 Cr3+(aq) + 7 H2O(l)
Which of the following statements is true?
A) Both Sn2+ and H+ are oxidizing agents.
B) Cr2O72- is the oxidizing agent.
C) Sn2+ is reduced.
D) The acid isn't important to the reaction.
E) None of the above are true.
3 Sn2+(aq) + Cr2O72-(aq) + 14 H+(aq) 3 Sn4+(aq) + 2 Cr3+(aq) + 7 H2O(l)
Which of the following statements is true?
A) Both Sn2+ and H+ are oxidizing agents.
B) Cr2O72- is the oxidizing agent.
C) Sn2+ is reduced.
D) The acid isn't important to the reaction.
E) None of the above are true.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
28
Use a table of standard reduction potentials to determine which of the following is the strongest reducing agent.
A) Co
B) Ca2+
C) Co3+
D) Cr
E) Au
A) Co
B) Ca2+
C) Co3+
D) Cr
E) Au

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
29
Given the following half-cell reduction potentials, determine which of the following is the strongest reducing agent.
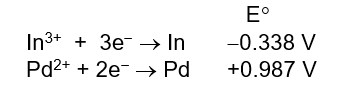
A) In3+
B) In
C) Pd2+
D) Pd
E) Cannot be determined from cell potentials alone
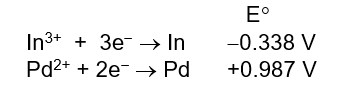
A) In3+
B) In
C) Pd2+
D) Pd
E) Cannot be determined from cell potentials alone

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
30
Use the following half reactions and accompanying standard reduction potentials to determine the best reducing agent. 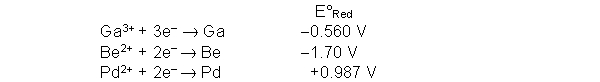
A) Ga3+
B) Be2+
C) Be
D) Pd2+
E) Pd
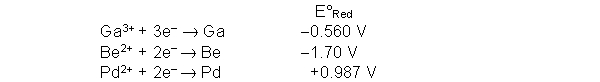
A) Ga3+
B) Be2+
C) Be
D) Pd2+
E) Pd

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
31
Given the following half-cell reduction potentials, determine which metal is the best reducing agent.
Co2+ + 2e - Co E = -0.28 V
= -0.28 V
Pd2+ + 2e - Pd E 11ee9f07_2055_deec_9fac_e13ec1386541_TB9692_11 = +0.987 V
Cd2+ + 2e - Cd E 11ee9f07_2055_deec_9fac_e13ec1386541_TB9692_11 = -0.403 V
A) Co
B) Pd
C) Cd
D) None would be a good reducing agent.
Co2+ + 2e - Co E
 = -0.28 V
= -0.28 VPd2+ + 2e - Pd E 11ee9f07_2055_deec_9fac_e13ec1386541_TB9692_11 = +0.987 V
Cd2+ + 2e - Cd E 11ee9f07_2055_deec_9fac_e13ec1386541_TB9692_11 = -0.403 V
A) Co
B) Pd
C) Cd
D) None would be a good reducing agent.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
32
Given the following redox reactions and cell potentials, rank from weakest to strongest the relative strengths of Al, Fe, and Cr as reducing agents. Al(s) + Cr3+(aq) Al3+(aq) + Cr(s) Eo = +0.966 V
Fe(s) + Cr3+(aq) Fe3+(aq) + Cr(s) Eo = -0.70 V
A) Cr < Fe < Al
B) Al < Fe < Cr
C) Al < Cr < Fe
D) Fe < Cr < Al
E) Fe < Al < Cr
Fe(s) + Cr3+(aq) Fe3+(aq) + Cr(s) Eo = -0.70 V
A) Cr < Fe < Al
B) Al < Fe < Cr
C) Al < Cr < Fe
D) Fe < Cr < Al
E) Fe < Al < Cr

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
33
Use a table of standard reduction potentials to determine which of the following is the strongest oxidizing agent.
A) H2O2 in base
B) H2O2 in acid
C) O2 in acid
D) CrO42- in acid
E) Br2
A) H2O2 in base
B) H2O2 in acid
C) O2 in acid
D) CrO42- in acid
E) Br2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
34
Use a table of standard reduction potentials to determine which is the strongest oxidizing agent among the following.
A) Cl2 gas at 1 atm pressure
B) O2 gas at 1 atm pressure in contact with 1 M acid
C) F2 gas at 1 atm pressure
D) Ag metal
E) H2O2 in acid solution
A) Cl2 gas at 1 atm pressure
B) O2 gas at 1 atm pressure in contact with 1 M acid
C) F2 gas at 1 atm pressure
D) Ag metal
E) H2O2 in acid solution

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following pairs of ions can't coexist in aqueous solution under standard-state conditions because a spontaneous redox reaction occurs?
A) Sn4+ and Fe3+
B) Sn4+ and Fe2+
C) Sn2+ and Fe3+
D) Sn2+ and Fe2+
E) None of these pairs of ions can coexist.
A) Sn4+ and Fe3+
B) Sn4+ and Fe2+
C) Sn2+ and Fe3+
D) Sn2+ and Fe2+
E) None of these pairs of ions can coexist.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
36
Which is the strongest oxidizing agent?
A) Ce4+
B) Ce3+
C) H+
D) Cr2+
E) Mg
A) Ce4+
B) Ce3+
C) H+
D) Cr2+
E) Mg

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following reagents should react with H+ to produce H2?
A) either Mg2+ and Cr2+
B) either Pd and Cr2+
C) either Pd2+
D) either Mg and Cr
E) Mg, Cr, and Pd
A) either Mg2+ and Cr2+
B) either Pd and Cr2+
C) either Pd2+
D) either Mg and Cr
E) Mg, Cr, and Pd

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following pairs of ions cannot coexist in solution because a spontaneous redox reaction occurs?
A) Cu2+ and Zn2+
B) Fe3+ and Hg22+
C) Cu2+ and I-
D) Al3+ and S2-
E) All of these pairs of ions can coexist.
A) Cu2+ and Zn2+
B) Fe3+ and Hg22+
C) Cu2+ and I-
D) Al3+ and S2-
E) All of these pairs of ions can coexist.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
39
Cobalt(III) oxide reacts with hydrogen gas to form cobalt metal and water.
Co2O3(s) + 3 H2(g) 2 Co(s) + 3 H2O(g)
What does this tell you about the relative strength of the oxidizing and reducing agents in this reaction?
A) The Co3+ ion is a stronger reducing agent than cobalt metal.
B) Cobalt metal is a better reducing agent than water.
C) Cobalt metal is a weaker reducing agent than hydrogen.
D) The Co3+ ion is a weaker oxidizing agent than water.
E) Statements (a) through (d) above are all false.
Co2O3(s) + 3 H2(g) 2 Co(s) + 3 H2O(g)
What does this tell you about the relative strength of the oxidizing and reducing agents in this reaction?
A) The Co3+ ion is a stronger reducing agent than cobalt metal.
B) Cobalt metal is a better reducing agent than water.
C) Cobalt metal is a weaker reducing agent than hydrogen.
D) The Co3+ ion is a weaker oxidizing agent than water.
E) Statements (a) through (d) above are all false.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
40
The equilibrium constant at 298 K for the following reaction is 1.2 x 105.
H2(g) + Sn4+(aq) 2 H+(aq) + Sn2+(aq)
What is the value of E° for the reaction?
A) -0.15 V
B) 0.15 V
C) 0.30 V
D) 0.35 V
E) 0.45 V
H2(g) + Sn4+(aq) 2 H+(aq) + Sn2+(aq)
What is the value of E° for the reaction?
A) -0.15 V
B) 0.15 V
C) 0.30 V
D) 0.35 V
E) 0.45 V

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
41
The equilibrium constant at 25 oC for the following reaction is 2 x 10-3.
H2(g) + 2Ru3+(aq) 2 H+(aq) + 2Ru2+(aq)
What is the value of E° for the reaction?
A) -0.16 V
B) 0.16 V
C) 1.00 V
D) 0.00 V
E) -0.08 V
H2(g) + 2Ru3+(aq) 2 H+(aq) + 2Ru2+(aq)
What is the value of E° for the reaction?
A) -0.16 V
B) 0.16 V
C) 1.00 V
D) 0.00 V
E) -0.08 V

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the value of the Ksp for CdS at 25 °C from the following data.
CdS + 2 e- Cd + S2- E° = -1.21 V
Cd + S2- E° = -1.21 V
Cd2+ + 2 e- Cd E° = -0.40 V
Cd E° = -0.40 V
A) 3 x 10-55
B) 4 x 10-28
C) 2 x 10-14
D) 3 x 1027
E) 6 x 10
CdS + 2 e-
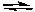 Cd + S2- E° = -1.21 V
Cd + S2- E° = -1.21 VCd2+ + 2 e-
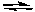 Cd E° = -0.40 V
Cd E° = -0.40 VA) 3 x 10-55
B) 4 x 10-28
C) 2 x 10-14
D) 3 x 1027
E) 6 x 10

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
43
There are two half-reactions for the reduction of O2 to H2O.
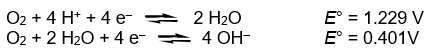
Use these half-reactions to calculate the ionization constant for water, Kw,
at 25 °C.
H2O(l) H+(aq) + OH-(aq) Kw = ?
H+(aq) + OH-(aq) Kw = ?
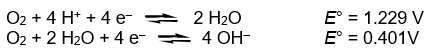
Use these half-reactions to calculate the ionization constant for water, Kw,
at 25 °C.
H2O(l)
 H+(aq) + OH-(aq) Kw = ?
H+(aq) + OH-(aq) Kw = ?
Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
44
The half-reaction reduction potentials for the mercury(I) and silver(I) ions are given below.
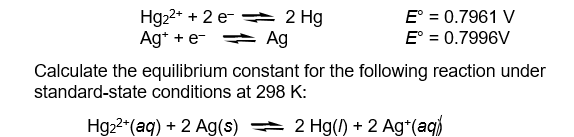


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the complex dissociation equilibrium constant (Kd) for the Cd(NH3)42+ complex from the following data at 298K. 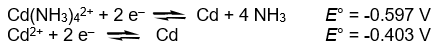
A) 2.7 x 10-7
B) 0.19
C) 6.6
D) 3.6 x 106
E) none of the above
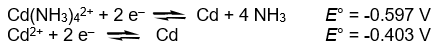
A) 2.7 x 10-7
B) 0.19
C) 6.6
D) 3.6 x 106
E) none of the above

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate the weight of sodium metal that would be produced by the electrolysis of molten sodium chloride for 1.00 hour with a 10.0-amp current.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
47
What is the ratio of the weight of Cl2 produced at the anode to the weight of Al produced at the cathode when 5 moles of electrons are passed through a molten sample of AlCl3?
A) about 2 g Cl2 per 1 g Al
B) about 4 g Cl2 per 1 g Al
C) about 8 g Cl2 per 1 g Al
D) about 16 g Cl2 per 1 g Al
E) none of the above
A) about 2 g Cl2 per 1 g Al
B) about 4 g Cl2 per 1 g Al
C) about 8 g Cl2 per 1 g Al
D) about 16 g Cl2 per 1 g Al
E) none of the above

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the amount of aluminum produced in 1.00 hour by the electrolysis of molten AlCl3 if the current is 10.0 A.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
49
Suppose a beer can weighs 40.0 g. Determine the amount of time in hours that a current of 100.0 amp need to be passed through a molten AlF3 electrolysis cell to produce enough Al to replace a discarded beer can. Sketch the electrolysis cell, labeling the electrodes and showing the direction of electron flow in the external circuit as part of your answer.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following electrolysis processes will produce the largest volume of Cl2 gas at STP?
A) passing 35 amps for 20 hours through an aqueous Na2SO4 solution
B) passing 10 amps for 2.69 hours through a 1 M NaCl solution
C) passing 2 moles electrons through a 1 M CaCl2 solution
D) passing 1.5 moles electrons through a 5 M CaCl2 solution
E) passing 1.4 moles electrons through a 5 M AlCl3 solution
A) passing 35 amps for 20 hours through an aqueous Na2SO4 solution
B) passing 10 amps for 2.69 hours through a 1 M NaCl solution
C) passing 2 moles electrons through a 1 M CaCl2 solution
D) passing 1.5 moles electrons through a 5 M CaCl2 solution
E) passing 1.4 moles electrons through a 5 M AlCl3 solution

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
51
A current of 1.5 amps is applied to a 1.00 L solution of 0.100 M hydrochloric acid for 1.0 hr. What is the pH of the solution after electrolysis is complete?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
52
A molten sample of TiCl4 was electrolyzed for 10.0 hours at 12 amps. What is the ratio of the weight of Cl2 produced compared to that of Ti?
A) 0.34 g Cl2/1.00 g Ti
B) 0.68 g Cl2/1.00 g Ti
C) 0.74 g Cl2/1.00 g Ti
D) 1.48 g Cl2/1.00 g Ti
E) 2.96 g Cl2/1.00 g Ti
A) 0.34 g Cl2/1.00 g Ti
B) 0.68 g Cl2/1.00 g Ti
C) 0.74 g Cl2/1.00 g Ti
D) 1.48 g Cl2/1.00 g Ti
E) 2.96 g Cl2/1.00 g Ti

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
53
An electric current is passed through a solution of CuSO4(aq) producing Cu(s) at the cathode and O2(g) at the anode. If 3.48 L of O2(g) measured at STP is produced at the anode, how many grams of Cu(s) must have been deposited on the cathode?
A) 3.48 g
B) 4.93 g
C) 9.87 g
D) 19.7 g
E) none of these
A) 3.48 g
B) 4.93 g
C) 9.87 g
D) 19.7 g
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
54
What is the ratio by weight of Br2 to Cr if a molten sample of CrBr2 is electrolyzed for 4.00 hr. at 10.0 amps?
A) 1.05 g Br2/1 g Cr
B) 1.54 g Br2/1 g Cr
C) 2.05 g Br2/1 g Cr
D) 3.07 g Br2/1 g Cr
E) 4.61 g Br2/1 g Cr
A) 1.05 g Br2/1 g Cr
B) 1.54 g Br2/1 g Cr
C) 2.05 g Br2/1 g Cr
D) 3.07 g Br2/1 g Cr
E) 4.61 g Br2/1 g Cr

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
55
How much Cl2 gas would be collected when a 2.00 M NaCl(aq) solution is electrolyzed for 2.00 hours with a current of 15.0 amps?
A) 0.0220 g
B) 39.7 g
C) 79.4 g
D) 159 g
E) none of the above
A) 0.0220 g
B) 39.7 g
C) 79.4 g
D) 159 g
E) none of the above

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
56
A current of 10.0 amps over a period of 3.00 hr is passed through a solution of molten KCl. What weight of K metal is produced?
A) 0.365 g
B) 0.729 g
C) 21.9 g
D) 43.8 g
E) 87.5 g
A) 0.365 g
B) 0.729 g
C) 21.9 g
D) 43.8 g
E) 87.5 g

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
57
A current of 2.0 amps passed through a molten solution of the salt MCl2 for a period of 30 minutes produces 1.32 g of Cl2 gas and 0.453 g of M. Which of the following metals is present in this compound?
A) Mg
B) Ca
C) Zn
D) Sr
E) Hg
A) Mg
B) Ca
C) Zn
D) Sr
E) Hg

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
58
What is the oxidation state of the osmium atom in an unknown salt if 26.7 grams of osmium plate out when a current of 15.0 amps is passed through a solution of this salt for 1.00 hour?
A) Os+
B) Os2+
C) Os3+
D) Os4+
E) Os5+
A) Os+
B) Os2+
C) Os3+
D) Os4+
E) Os5+

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
59
What is the oxidation state of the cerium atom in CexZy if a current of 1.2 amps for 3.0 hours deposits 4.70 g of Ce at the cathode?
A) 0
B) +1
C) +2
D) +3
E) +4
A) 0
B) +1
C) +2
D) +3
E) +4

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
60
What is the oxidation state of the tin atom in an unknown salt if 14.8 grams of tin are plated out when a current of 40 amps is passed through a molten solution of this salt for 10 minutes?
A) +1
B) +2
C) +3
D) +4
E) +6
A) +1
B) +2
C) +3
D) +4
E) +6

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is the correct half-cell reaction for the anode process in the electrolysis of an aqueous solution of potassium sulfate?
A) SO42-(aq) SO2(g) + O2(g) + 2 e-
B) H2O(l) 1/2 O2(g) + 2 H+(aq) + 2 e-
C) H2O(l) + 1/2 O2(g) + 2 e- 2 OH-(aq)
D) SO42-(aq) + 4 H+(aq) + 2 e- SO2(g) + 2 H2O(l)
E) 3 H2O(l) + e- 1/2 H2(g) + 3 OH-(aq) + 2 H+(aq)
A) SO42-(aq) SO2(g) + O2(g) + 2 e-
B) H2O(l) 1/2 O2(g) + 2 H+(aq) + 2 e-
C) H2O(l) + 1/2 O2(g) + 2 e- 2 OH-(aq)
D) SO42-(aq) + 4 H+(aq) + 2 e- SO2(g) + 2 H2O(l)
E) 3 H2O(l) + e- 1/2 H2(g) + 3 OH-(aq) + 2 H+(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following statements about the electrolysis of an aqueous solution of NaCl is true?
A) O2 is liberated at the cathode.
B) H2 is liberated at the cathode.
C) Na is liberated at the cathode.
D) Cl2 is liberated at the cathode.
E) The decomposition potential must not be exceeded.
A) O2 is liberated at the cathode.
B) H2 is liberated at the cathode.
C) Na is liberated at the cathode.
D) Cl2 is liberated at the cathode.
E) The decomposition potential must not be exceeded.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
63
In the electrolysis of NaBr(aq), which of the following products is least likely to be found?
A) H2(g)
B) O2(g)
C) Br2(l)
D) Na(s)
E) NaOH(aq)
A) H2(g)
B) O2(g)
C) Br2(l)
D) Na(s)
E) NaOH(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
64
What is the correct coefficient for Na2MnO4 in the balanced equation for the air oxidation of manganese dioxide to sodium manganate in the presence of sodium carbonate?
MnO2(s) + Na2CO3(aq) + O2(g) CO2(g) + Na2MnO4(aq)
A) 1
B) 3/2
C) 2
D) 5/2
E) 3
MnO2(s) + Na2CO3(aq) + O2(g) CO2(g) + Na2MnO4(aq)
A) 1
B) 3/2
C) 2
D) 5/2
E) 3

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
65
What is the coefficient for sulfur in the following redox reaction?
S(s) + KClO3(s) SO2(g) + KCl(s) + bang!
A) 1
B) 2
C) 3
D) 4
E) 5
S(s) + KClO3(s) SO2(g) + KCl(s) + bang!
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
66
The following equation, when balanced, involves a total of ___ electrons.
ClO3-(aq) + I2(aq) + H2O(l) IO3-(aq) + Cl-(aq) + H+(aq)
A) 5
B) 6
C) 12
D) 24
E) 30
ClO3-(aq) + I2(aq) + H2O(l) IO3-(aq) + Cl-(aq) + H+(aq)
A) 5
B) 6
C) 12
D) 24
E) 30

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
67
Complete and balance the following equation:
PbO2(s) + Cl-(aq) + H+(aq) Pb2+(aq) + Cl2(aq)
PbO2(s) + Cl-(aq) + H+(aq) Pb2+(aq) + Cl2(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
68
refer to the following reaction in acid solution.
CuS(s) + NO3-(aq)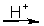 Cu2+(aq) + SO42-(aq) + NO(g)
Cu2+(aq) + SO42-(aq) + NO(g)
-In the balanced half-reaction for the NO3- ion, how many electrons are involved?
A) 1
B) 2
C) 3
D) 4
E) none of these
CuS(s) + NO3-(aq)
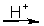 Cu2+(aq) + SO42-(aq) + NO(g)
Cu2+(aq) + SO42-(aq) + NO(g)-In the balanced half-reaction for the NO3- ion, how many electrons are involved?
A) 1
B) 2
C) 3
D) 4
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following isn't true?
A) The reducing agent is CuS.
B) The CuS is oxidized.
C) The NO3- ion is the oxidizing agent.
D) The NO3- ion is reduced.
E) The oxidation number of the copper changes from 0 to +2.
A) The reducing agent is CuS.
B) The CuS is oxidized.
C) The NO3- ion is the oxidizing agent.
D) The NO3- ion is reduced.
E) The oxidation number of the copper changes from 0 to +2.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
70
Write a balanced chemical equation for the following reaction.
MnO2(s) + PbO2(s) + H+(aq) MnO4-(aq) + Pb2+(aq)
MnO2(s) + PbO2(s) + H+(aq) MnO4-(aq) + Pb2+(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
71
refer to the following incomplete, unbalanced equation
Cr2O72-(aq) + NO(g)
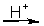 Cr3+(aq) + NO3-(aq)
Cr3+(aq) + NO3-(aq)
-How many electrons are in the half-reaction involving Cr2O72-?
A) 2
B) 3
C) 4
D) 5
E) 6
Cr2O72-(aq) + NO(g)
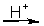 Cr3+(aq) + NO3-(aq)
Cr3+(aq) + NO3-(aq)-How many electrons are in the half-reaction involving Cr2O72-?
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
72
refer to the following incomplete, unbalanced equation
Cr2O72-(aq) + NO(g)
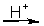 Cr3+(aq) + NO3-(aq)
Cr3+(aq) + NO3-(aq)
-What is the coefficient of the NO in the balanced equation?
A) 1
B) 2
C) 3
D) 4
E) 6
Cr2O72-(aq) + NO(g)
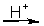 Cr3+(aq) + NO3-(aq)
Cr3+(aq) + NO3-(aq)-What is the coefficient of the NO in the balanced equation?
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
73
Write a balanced chemical equation for the following reaction, which can be used to standardize aqueous permanganate ion solutions.
H2C2O4(aq) + MnO4-(aq) + H+(aq) CO2(g) + Mn2+(aq)
H2C2O4(aq) + MnO4-(aq) + H+(aq) CO2(g) + Mn2+(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
74
Balance the following redox reaction. Clearly show the two balanced half-reactions and indicate which represents oxidation and which reduction.
H2O2(aq) + NO(g) + H+(aq) NO3-(aq)
H2O2(aq) + NO(g) + H+(aq) NO3-(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
75
Balance the following oxidation-reduction equation.
HI(aq) + HNO3(aq) NO(g) + I2(aq)
HI(aq) + HNO3(aq) NO(g) + I2(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
76
Balance the following oxidation-reduction equation.
CrO42-(aq) + HSnO2-(aq)
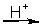
HSnO3-(aq) + CrO2-(aq)
CrO42-(aq) + HSnO2-(aq)
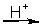
HSnO3-(aq) + CrO2-(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
77
Redox Reactions in Basic Solutions
refer to the following reaction which occurs in basic solution:
OCl-(aq) ClO2-(aq) + Cl2(aq)
-How many OCl- ions are consumed in the balanced equation for this reaction?
A) 2
B) 3
C) 4
D) 5
E) 6
refer to the following reaction which occurs in basic solution:
OCl-(aq) ClO2-(aq) + Cl2(aq)
-How many OCl- ions are consumed in the balanced equation for this reaction?
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
78
Redox Reactions in Basic Solutions
refer to the following reaction which occurs in basic solution:
OCl-(aq) ClO2-(aq) + Cl2(aq)
-How many OH- ions are involved in the simplest balanced equation for this reaction?
A) 0
B) 1
C) 2
D) 3
E) 4
refer to the following reaction which occurs in basic solution:
OCl-(aq) ClO2-(aq) + Cl2(aq)
-How many OH- ions are involved in the simplest balanced equation for this reaction?
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
79
Redox Reactions in Basic Solutions
refer to the following reaction which occurs in basic solution:
OCl-(aq) ClO2-(aq) + Cl2(aq)
-What is the net effect on the OH- ion concentration in this reaction?
A) The OH- ion concentration increases.
B) The OH- ion concentration decreases.
C) The OH- ion concentration remains the same.
D) There is no way to predict what happens to the OH- ion concentration in this reaction.
refer to the following reaction which occurs in basic solution:
OCl-(aq) ClO2-(aq) + Cl2(aq)
-What is the net effect on the OH- ion concentration in this reaction?
A) The OH- ion concentration increases.
B) The OH- ion concentration decreases.
C) The OH- ion concentration remains the same.
D) There is no way to predict what happens to the OH- ion concentration in this reaction.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
80
refer to the following reaction which occurs in basic solution.
CrO42- + PH3 Cr(OH)4- + P4
-Which of the following are oxidizing agents in the forward and reverse directions, respectively?
A) CrO42- and PH3
B) CrO42- and P4
C) Cr(OH)4- and PH3
D) Cr(OH)4- and P4
E) CrO42- and OH-
CrO42- + PH3 Cr(OH)4- + P4
-Which of the following are oxidizing agents in the forward and reverse directions, respectively?
A) CrO42- and PH3
B) CrO42- and P4
C) Cr(OH)4- and PH3
D) Cr(OH)4- and P4
E) CrO42- and OH-

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck



