Deck 4: The Covalent Bond
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/105
Play
Full screen (f)
Deck 4: The Covalent Bond
1
Which of the following elements has five valence electrons?
A) Sr
B) K
C) Si
D) Se
E) As
A) Sr
B) K
C) Si
D) Se
E) As
As
2
Which of the following elements has 2 valence electrons?
A) Ca
B) Ga
C) Ge
D) As
E) Se
A) Ca
B) Ga
C) Ge
D) As
E) Se
Ca
3
How many valence electrons are in the IF2- ion?
A) 7
B) 14
C) 20
D) 21
E) 22
A) 7
B) 14
C) 20
D) 21
E) 22
22
4
Which of the following species has all its electrons paired?
A) Fe3+
B) Zn2+
C) ClO2
D) NO
E) NO2
A) Fe3+
B) Zn2+
C) ClO2
D) NO
E) NO2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
5
How many of nonbonding electrons are in the valence shell of the iodine atom in the IF2- ion?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
6
How many of nonbonding electrons are in the valence shell of the iodine atom in the ICl4- ion?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
7
How many nonbonding are on the central atom in the Lewis structure of xenon tetrafluoride?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
8
How many of bonding electrons can be found in the valence shell of
The nitrogen atom in HNO2? (Hint: the skeleton structure is HONO)
A) 0
B) 1
C) 2
D) 3
E) 4
The nitrogen atom in HNO2? (Hint: the skeleton structure is HONO)
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
9
How many of nonbonding electrons can be found on the chlorine atom in chlorous acid, HOClO?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following elements would form an XF62- ion that has no nonbonding electrons in the valence shell of the central atom?
A) Ca
B) C
C) Si
D) S
E) P
A) Ca
B) C
C) Si
D) S
E) P

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
11
Nitrous oxide (N2O) is an anesthetic commonly known as "laughing gas." Which of the following is a correct Lewis structure for this compound?
A)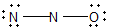
B)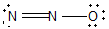
C)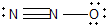
D)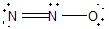
E)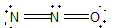
A)
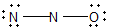
B)
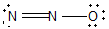
C)
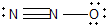
D)
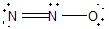
E)
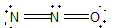

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
12
Which element would form XF5 for which there are no nonbonding electrons in the Lewis structure?
A) N
B) Al
C) Si
D) P
E) Br
A) N
B) Al
C) Si
D) P
E) Br

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
13
If X is a 3rd row element and XCl3 has the Lewis structure 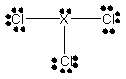
X must be:
A) Al
B) Si
C) P
D) S
E) Cl

X must be:
A) Al
B) Si
C) P
D) S
E) Cl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
14
If the XF6- ion has the Lewis structure
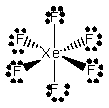
X could be which of the following elements?
A) N
B) Al
C) Si
D) P
E) Br

X could be which of the following elements?
A) N
B) Al
C) Si
D) P
E) Br

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
15
Assume that the XF3 molecule has the following Lewis structure.
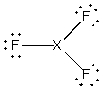
The molecule could be:
A) boron trifluoride, i.e., X = B
B) nitrogen trifluoride, i.e., X = N
C) chlorine trifluoride, i.e., X = Cl
D) phosphorus trifluoride, i.e., X = P
E) more than one of the above

The molecule could be:
A) boron trifluoride, i.e., X = B
B) nitrogen trifluoride, i.e., X = N
C) chlorine trifluoride, i.e., X = Cl
D) phosphorus trifluoride, i.e., X = P
E) more than one of the above

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following elements would form a compound with the following Lewis structure?

A) Al
B) Si
C) P
D) S
E) Cl

A) Al
B) Si
C) P
D) S
E) Cl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
17
If there are two pairs of nonbonding electrons in the valence shell of the central atom in the XF4- ion, in which Group of the periodic table does the element X belong?
A) Group IVA
B) Group VA
C) Group VIA
D) Group VIIA
E) Group VIIIA
A) Group IVA
B) Group VA
C) Group VIA
D) Group VIIA
E) Group VIIIA

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
18
What is element X in the following Lewis structure?
A) B
B) C
C) N
D) O
E) F

A) B
B) C
C) N
D) O
E) F

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following species contains only single bonds?
A) CN-
B) NO+
C) CO
D) O22-
E) Cl2CO
A) CN-
B) NO+
C) CO
D) O22-
E) Cl2CO

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following would be the best Lewis structure for NOCl?
A)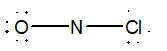
B)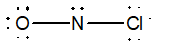
C)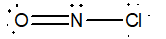
D)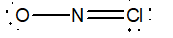
E)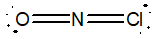
A)
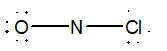
B)
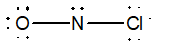
C)
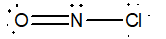
D)
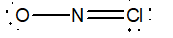
E)
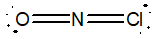

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following compounds would have the same Lewis structure as the N2 molecule?
(I) F2 (II) CO (III) NO (IV) CN- (V) NO+
A) I
B) II and IV
C) II and III
D) II, IV and V
E) IV and V
(I) F2 (II) CO (III) NO (IV) CN- (V) NO+
A) I
B) II and IV
C) II and III
D) II, IV and V
E) IV and V

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
22
The correct Lewis structure for CH2Cl2 contains:
A) 2 single bonds, 2 double bonds and no nonbonding electrons
B) 4 single bonds and no nonbonding electrons
C) 4 single bonds and four nonbonding electrons
D) 2 single bonds, 2 double bonds, and eight nonbonding electrons
E) 4 single bonds and 12 nonbonding electrons
A) 2 single bonds, 2 double bonds and no nonbonding electrons
B) 4 single bonds and no nonbonding electrons
C) 4 single bonds and four nonbonding electrons
D) 2 single bonds, 2 double bonds, and eight nonbonding electrons
E) 4 single bonds and 12 nonbonding electrons

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
23
The correct Lewis structure for the NO2+ ion has:
A) 2 double bonds and eight nonbonding electrons
B) 1 double bond, 1 single bond, and 12 nonbonding electrons
C) 2 single bonds and 12 nonbonding electrons
D) 2 triple bonds and 4 nonbonding electrons
E) 1 single bond, 1 double bond, and 8 nonbonding electrons
A) 2 double bonds and eight nonbonding electrons
B) 1 double bond, 1 single bond, and 12 nonbonding electrons
C) 2 single bonds and 12 nonbonding electrons
D) 2 triple bonds and 4 nonbonding electrons
E) 1 single bond, 1 double bond, and 8 nonbonding electrons

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
24
In which Group of the periodic table does element X belong if there are one pair of nonbonding electrons in the valence shell of the central atom in the XF4- ion?
A) Group IIIA
B) Group IVA
C) Group VA
D) Group VIA
E) none of these
A) Group IIIA
B) Group IVA
C) Group VA
D) Group VIA
E) none of these

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
25
Which element forms a compound with the following Lewis structure?
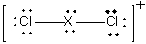
A) Al
B) Si
C) P
S) S
E) I
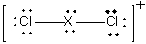
A) Al
B) Si
C) P
S) S
E) I

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following are exceptions to the Lewis octet rule?
(I) CO2 (II) BeF2 (III) SF4 (IV) SO3 (V) PCl3
A) I and II
B) II and III
C) III and IV
D) I, II and III
E) none of these are exceptions to the Lewis octet rule
(I) CO2 (II) BeF2 (III) SF4 (IV) SO3 (V) PCl3
A) I and II
B) II and III
C) III and IV
D) I, II and III
E) none of these are exceptions to the Lewis octet rule

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following are exceptions to the Lewis octet rule?
(I) BF3 (II) H2CO (III) XeF4 (IV) H3O+ (V) ClF3
A) I and II
B) I and III
C) I, III and V
D) II and III
E) III and V
(I) BF3 (II) H2CO (III) XeF4 (IV) H3O+ (V) ClF3
A) I and II
B) I and III
C) I, III and V
D) II and III
E) III and V

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following elements is the most likely to follow the octet rule?
A) H
B) C
C) Si
D) As
E) All of these elements must follow the octet rule.
A) H
B) C
C) Si
D) As
E) All of these elements must follow the octet rule.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the bonds in ethanol, CH3CH2OH, would have a bond length of approximately 0.103 nm?
Covalent Radii: H = 0.037 nm, C = 0.077 nm, O = 0.066 nm
A) C-C
B) C-O
C) C-H
D) O-H
E) not enough information is given
Covalent Radii: H = 0.037 nm, C = 0.077 nm, O = 0.066 nm
A) C-C
B) C-O
C) C-H
D) O-H
E) not enough information is given

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds/ions has the shortest C-O bond length?
A) CH3OH
B) H2CO
C) CO32-
D) CH3CH2OH
E) all C-O bonds are the same length
A) CH3OH
B) H2CO
C) CO32-
D) CH3CH2OH
E) all C-O bonds are the same length

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following bond lengths is the largest?
A) H-C
B) H-S
C) H-O
D) H-F
E) H-H
A) H-C
B) H-S
C) H-O
D) H-F
E) H-H

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
32
Which bond length is the shortest in the following compound?
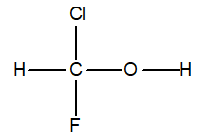
A) HF-C
B) Cl-C
C) F-C
D) C-O
E) O-H

A) HF-C
B) Cl-C
C) F-C
D) C-O
E) O-H

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
33
In which of the following is the carbon to oxygen bond the shortest?
A)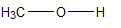
B)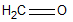
C)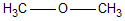
D)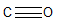
E) all C-O bonds are the same length
A)
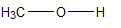
B)
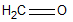
C)
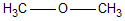
D)
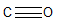
E) all C-O bonds are the same length

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds contains the strongest carbon-nitrogen bond? (Hint: Look at their Lewis structures)
A) H2CNH
B) HCN
C) H3CNH2
D) H3CNO
E) all of these molecules contain C-N single bonds
A) H2CNH
B) HCN
C) H3CNH2
D) H3CNO
E) all of these molecules contain C-N single bonds

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following molecules or ions is not a resonance hybrid?
A) SCN-
B) CO32-
C) C2H4
D) N2O
E) all are resonance hybrids
A) SCN-
B) CO32-
C) C2H4
D) N2O
E) all are resonance hybrids

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
36
What is the difference between an isomer and a resonance structure?

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following would be a resonance structure for the compound shown below.
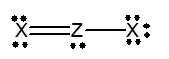
A)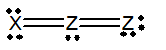
B)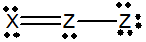
C)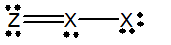
D)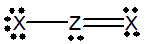
E) none of these
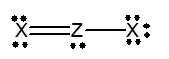
A)
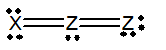
B)
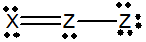
C)
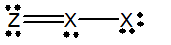
D)
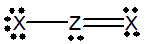
E) none of these

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules is best described with two resonance structures?
A) NO2-
B) H2O
C) OF2
D) ClNO
E) BeF2
A) NO2-
B) H2O
C) OF2
D) ClNO
E) BeF2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following isn't a resonance hybrid?
A) CO2
B) NO2-
C) N2O
D) SO3
E) SCN-
A) CO2
B) NO2-
C) N2O
D) SO3
E) SCN-

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following are resonance hybrids?
(I) HCO2- (II) PH3 (III) HCN (IV) SO42- (V) NO2
A) I
B) II and IV
C) IV and V
D) I and IV
E) I and V
(I) HCO2- (II) PH3 (III) HCN (IV) SO42- (V) NO2
A) I
B) II and IV
C) IV and V
D) I and IV
E) I and V

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following can be used to predict an element's electronegativity?
A) average valence electron energy
B) Lewis Structures
C) bond angles
D) bond length
E) resonance
A) average valence electron energy
B) Lewis Structures
C) bond angles
D) bond length
E) resonance

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following elements has the greatest electronegativity?
A) Mg
B) Cu
C) S
D) As
E) Sr
A) Mg
B) Cu
C) S
D) As
E) Sr

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following lists the elements in the correct order of increasing electronegativity?
A) Si < S < O
B) S < Si < O
C) S < O < Si
D) Si < O < S
E) O < Si < S
A) Si < S < O
B) S < Si < O
C) S < O < Si
D) Si < O < S
E) O < Si < S

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is the best true description of the charge on an atom within a molecule?
A) oxidation number
B) partial charge
C) formal charge
D) all of these are equally good
E) none of these
A) oxidation number
B) partial charge
C) formal charge
D) all of these are equally good
E) none of these

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
45
In which of the following compounds is the bond the least polar?
A) NaCl
B) CH4
C) CO
D) Cl2
E) HCl
A) NaCl
B) CH4
C) CO
D) Cl2
E) HCl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
46
Use formal charge to decide which of the following skeleton structures will give the best Lewis structure of the molecule. Show the values of formal charge and explain your choice of the best structure.



Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
47
The "zwitterion" form of the amino acid glycine is:
H3N+CH2CO2-. The properties of the various amino acids depend heavily on the presence (or absence) of charge on the side chain. Consider the amino acid arginine, for example, which has the following Lewis structure:
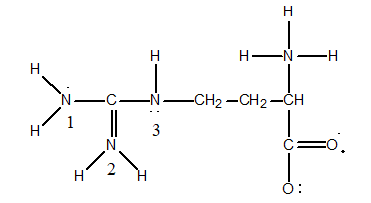 Determine the Formal Charge on the nitrogens labeled 1, 2 and 3.
Determine the Formal Charge on the nitrogens labeled 1, 2 and 3.
A) N(1) = 0, N(2) = +1, and N(3) = 0
B) N(1) = 0, N(2) = -1, and N(3) = 0
C) N(1) = +1, N(2) = 0, and N(3) = +1
D) N(1) = -1, N(2) = 0, and N(3) = -1
E) N(1) = +1, N(2) = 0, and N(3) = -1
H3N+CH2CO2-. The properties of the various amino acids depend heavily on the presence (or absence) of charge on the side chain. Consider the amino acid arginine, for example, which has the following Lewis structure:
 Determine the Formal Charge on the nitrogens labeled 1, 2 and 3.
Determine the Formal Charge on the nitrogens labeled 1, 2 and 3.A) N(1) = 0, N(2) = +1, and N(3) = 0
B) N(1) = 0, N(2) = -1, and N(3) = 0
C) N(1) = +1, N(2) = 0, and N(3) = +1
D) N(1) = -1, N(2) = 0, and N(3) = -1
E) N(1) = +1, N(2) = 0, and N(3) = -1

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
48
Our response to the anthrax scare was a 20-year-old antibacterial known as ciprofloxacin or "Cipro."
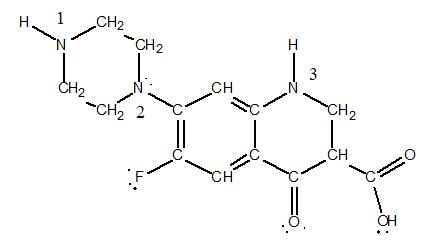
Apply the concept of formal charge to the three nitrogen atoms labeled 1, 2 and 3. Are they neutral, negatively charged, or positively charged?
A) N(1) = -1, N(2) = -1, and N(3) = -1
B) N(1) = -1, N(2) = 0, and N(3) = 0
C) N(1) = 0, N(2) = 0, and N(3) = 0
D) N(1) = 0, N(2) = +1, and N(3) = +1
E) N(1) = +1, N(2) = +1, and N(3) = +1

Apply the concept of formal charge to the three nitrogen atoms labeled 1, 2 and 3. Are they neutral, negatively charged, or positively charged?
A) N(1) = -1, N(2) = -1, and N(3) = -1
B) N(1) = -1, N(2) = 0, and N(3) = 0
C) N(1) = 0, N(2) = 0, and N(3) = 0
D) N(1) = 0, N(2) = +1, and N(3) = +1
E) N(1) = +1, N(2) = +1, and N(3) = +1

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
49
Which element will combine with sulfur to form a linear molecule with the formula XS2?
A) Li
B) B
C) C
D) O
E) F
A) Li
B) B
C) C
D) O
E) F

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
50
In which compound are the valence electrons on the sulfur arranged towards the corners of a tetrahedron?
A) SF3+
B) SF4
C) SF5
D) SF6
E) both SF4 and SF6
A) SF3+
B) SF4
C) SF5
D) SF6
E) both SF4 and SF6

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
51
What is the distribution of electrons around the S atom in H2SO4?
[Hint: The skeleton structure of this oxyacid is O2S(OH)2.
A) linear
B) trigonal planar
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
[Hint: The skeleton structure of this oxyacid is O2S(OH)2.
A) linear
B) trigonal planar
C) tetrahedral
D) trigonal bipyramidal
E) octahedral

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
52
What is the distribution of the oxygen atoms around the N atom in HNO3?
[Hint: The skeleton structure of this oxyacid is HONO2]
A) linear
B) trigonal planar
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
[Hint: The skeleton structure of this oxyacid is HONO2]
A) linear
B) trigonal planar
C) tetrahedral
D) trigonal bipyramidal
E) octahedral

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
53
The bond angles in most symmetric, ABx, molecules are the same. Which of the following is an exception to this rule?
A) NH3
B) SF6
C) PCl5
D) SO3
E) CO2
A) NH3
B) SF6
C) PCl5
D) SO3
E) CO2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
54
Which term best describes the shape of the I3- ion?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) trigonal bipyramidal
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) trigonal bipyramidal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
55
What is the molecular geometry of the compound formed by the reaction of nitrogen with fluorine?
A) bent
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) none of the above
A) bent
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) none of the above

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
56
What is the molecular geometry of the SF5+ ion?
A) see-saw
B) square planar
C) square pyramidal
D) trigonal bipyramidal
E) octahedral
A) see-saw
B) square planar
C) square pyramidal
D) trigonal bipyramidal
E) octahedral

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
57
What is the best description of the structure of the SF3+ ion?
A) bent or angular
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) trigonal bipyramidal
A) bent or angular
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) trigonal bipyramidal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
58
The molecular structure of the IO3- ion is best described as:
A) bent or angular
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) T-shaped
A) bent or angular
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) T-shaped

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following molecules has a trigonal planar shape?
A) BeCl2
B) PCl3
C) ICl2+
D) BF3
E) H3O+
A) BeCl2
B) PCl3
C) ICl2+
D) BF3
E) H3O+

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following molecules are tetrahedral?
(I) SiF4 (II) CH4 (III) NF4+ (IV) BF4- (V) TeF4
A) I and II
B) I, II and III
C) I, II, III and IV
D) II, III and IV
E) V
(I) SiF4 (II) CH4 (III) NF4+ (IV) BF4- (V) TeF4
A) I and II
B) I, II and III
C) I, II, III and IV
D) II, III and IV
E) V

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following molecules or ions doesn't have a tetrahedral shape?
A) SiF4
B) NF4+
C) BF4-
D) SeF4
E) CF4
A) SiF4
B) NF4+
C) BF4-
D) SeF4
E) CF4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following molecules or ions isn't planar?
A) NH3
B) BF3
C) CO32-
D) NO3-
E) none of these molecules or ions are planar
A) NH3
B) BF3
C) CO32-
D) NO3-
E) none of these molecules or ions are planar

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
63
What is the best description of the shape of the TeH2 molecules?
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) seesaw
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) seesaw

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
64
Metformin is the chemical name for a prescription drug sold as "glucophage," which is an oral hypoglycemic. In other words, a pill that lowers blood sugar levels so that adult on-set diabetics don't have to inject themselves with insulin. The Lewis structure of metformin is shown below.
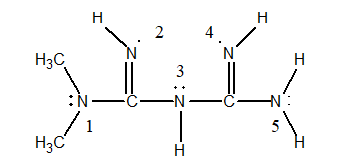
Use the electron domain model to predict the geometry around the nitrogen atom in the center of the molecule, e.g., N(3).
A) bent or angular
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) tetrahedral

Use the electron domain model to predict the geometry around the nitrogen atom in the center of the molecule, e.g., N(3).
A) bent or angular
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
65
Terpin hydrate is an expectorant that was used for many years by people with serious coughs, such as those suffering from pneumonia.
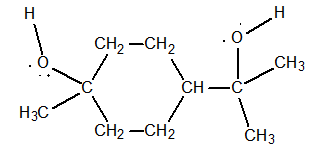
Use the electron domain model to predict the distribution of electron domains in the valence shell of the oxygen atoms in this compound.
A) linear
B) bent or angular
C) trigonal planar
D) tetrahedral
E) trigonal bipyramidal

Use the electron domain model to predict the distribution of electron domains in the valence shell of the oxygen atoms in this compound.
A) linear
B) bent or angular
C) trigonal planar
D) tetrahedral
E) trigonal bipyramidal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
66
When the rare gas elements (He, Ne, Ar, Kr, Xe and Rn) were first
Discovered, a sample of Ar was sent to Moissan, who had discovered the most active of the known elements: F2. The failure of Moissan's attempts to react argon with fluorine, coupled with repeated failures by other chemists to get the more abundant of these gases to undergo chemical reaction, eventually led to their being labeled inert gases. We now know this isn't so. Xe reacts with F2 to form a variety of compounds, including XeF4. Which of the following best describes the XeF4 molecule?
A) Trigonal bipyramidal distribution of valence electrons on the Xe with a T-shaped molecular geometry.
B) Trigonal bipyramidal distribution of valence electrons on the Xe with a trigonal planar molecular geometry.
C) Trigonal bipyramidal distribution of valence electrons on the Xe with a pyramidal molecular geometry.
D) Octahedral distribution of valence electrons on the Xe with a square planar molecular geometry.
E) Octahedral distribution of valence electrons on the Xe with a seesaw molecular geometry.
Discovered, a sample of Ar was sent to Moissan, who had discovered the most active of the known elements: F2. The failure of Moissan's attempts to react argon with fluorine, coupled with repeated failures by other chemists to get the more abundant of these gases to undergo chemical reaction, eventually led to their being labeled inert gases. We now know this isn't so. Xe reacts with F2 to form a variety of compounds, including XeF4. Which of the following best describes the XeF4 molecule?
A) Trigonal bipyramidal distribution of valence electrons on the Xe with a T-shaped molecular geometry.
B) Trigonal bipyramidal distribution of valence electrons on the Xe with a trigonal planar molecular geometry.
C) Trigonal bipyramidal distribution of valence electrons on the Xe with a pyramidal molecular geometry.
D) Octahedral distribution of valence electrons on the Xe with a square planar molecular geometry.
E) Octahedral distribution of valence electrons on the Xe with a seesaw molecular geometry.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
67
An element Z forms a molecular compound ZBr3 that is found to have trigonal pyramidal molecular geometry. The element Z must be in which group in the periodic table?
A) IIIA
B) IVA
C) VA
D) VIA
E) VIIA
A) IIIA
B) IVA
C) VA
D) VIA
E) VIIA

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
68
Tellurium hydride is one of the foulest smelling compounds known. How many pairs of nonbonding electrons are in the valence shell of the tellurium atom in TeH2?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
69
There is a single pair of nonbonding electrons on the central atom in the Lewis structure of ICl5. This molecule has:
A) a trigonal pyramidal distribution of electrons and an octahedral molecular geometry.
B) an octahedral distribution of electrons and a square pyramidal geometry.
C) a trigonal bipyramidal distribution of electrons and a seesaw molecular geometry.
D) an octahedral distribution of electrons and a trigonal bipyramidal geometry.
E) a trigonal bipyramidal distribution of electrons and trigonal bipyramidal geometry.
A) a trigonal pyramidal distribution of electrons and an octahedral molecular geometry.
B) an octahedral distribution of electrons and a square pyramidal geometry.
C) a trigonal bipyramidal distribution of electrons and a seesaw molecular geometry.
D) an octahedral distribution of electrons and a trigonal bipyramidal geometry.
E) a trigonal bipyramidal distribution of electrons and trigonal bipyramidal geometry.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following molecules are linear?
(I) C2H2 (II) CO2 (III) NO2- (IV) NO2+ (V) H2O
A) I and II
B) I, II and III
C) I, II and IV
D) II and III
E) V
(I) C2H2 (II) CO2 (III) NO2- (IV) NO2+ (V) H2O
A) I and II
B) I, II and III
C) I, II and IV
D) II and III
E) V

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following has a square-planar geometry?
A) SF4
B) ClO4-
C) CCl4
D) ICl4-
E) PO43-
A) SF4
B) ClO4-
C) CCl4
D) ICl4-
E) PO43-

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following species isn't planar?
A) SO3
B) SO32-
C) SO2
D) BF3
E) NO3-
A) SO3
B) SO32-
C) SO2
D) BF3
E) NO3-

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
73
What is the molecular geometry of xenon tetrafluoride?
A) tetrahedral
B) trigonal bipyramidal
C) seesaw
D) square planar
E) octahedral
A) tetrahedral
B) trigonal bipyramidal
C) seesaw
D) square planar
E) octahedral

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
74
The shape of the NO2- ion is best described as:
A) linear
B) T-shaped
C) bent
D) square planar
E) pyramidal
A) linear
B) T-shaped
C) bent
D) square planar
E) pyramidal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
75
How many pairs of nonbonding electrons are in the valence shell of the central iodine atom in the I3- ion?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
76
The Raschig process involves the reaction between ammonia and the hypochlorite ion to form hydrazine, which has been used as a rocket fuel.
2 NH3(aq) + OCl-(aq) H2NNH2(g) + Cl-(aq) +H2O(l)
There are isolated cases of individuals making hydrazine unexpectedly in their homes when cleaning a commode. They start with a cleaner that contains ammonia, and then, when this doesn't work, they mix it with a cleaner (such as Chlorox) that contains the hypochlorite ion. How many pairs of nonbonding electrons can be found on each nitrogen atom in the hydrazine molecule? (Note: Assume that there is a bond between the two nitrogen atoms in hydrazine.)
A) 0
B) 1
C) 2
D) 3
E) The two N atoms contain a different number of nonbonding electrons.
2 NH3(aq) + OCl-(aq) H2NNH2(g) + Cl-(aq) +H2O(l)
There are isolated cases of individuals making hydrazine unexpectedly in their homes when cleaning a commode. They start with a cleaner that contains ammonia, and then, when this doesn't work, they mix it with a cleaner (such as Chlorox) that contains the hypochlorite ion. How many pairs of nonbonding electrons can be found on each nitrogen atom in the hydrazine molecule? (Note: Assume that there is a bond between the two nitrogen atoms in hydrazine.)
A) 0
B) 1
C) 2
D) 3
E) The two N atoms contain a different number of nonbonding electrons.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following compounds/ions contains a bond angle of 120 11 ?
11 ?
A) CH3OH
B) CO32-
C) CO2
D) CH3Cl
E) CH2Cl2
 11 ?
11 ?A) CH3OH
B) CO32-
C) CO2
D) CH3Cl
E) CH2Cl2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
78
Estimate the hydrogen-phosphorus-hydrogen bond angle in PH3.
A) approximately 90
B) approximately 109
C) approximately 120 11ee9eaa_79d4_764b_9fac_596588eaddbc_TB9692_11
D) approximately 180 11ee9eaa_79d4_764b_9fac_596588eaddbc_TB9692_11
E) approximately 150 11ee9eaa_79d4_764b_9fac_596588eaddbc_TB9692_11
A) approximately 90

B) approximately 109

C) approximately 120 11ee9eaa_79d4_764b_9fac_596588eaddbc_TB9692_11
D) approximately 180 11ee9eaa_79d4_764b_9fac_596588eaddbc_TB9692_11
E) approximately 150 11ee9eaa_79d4_764b_9fac_596588eaddbc_TB9692_11

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
79
Which one of the following molecular geometries has bond angles which are all equal?
A) tetrahedral
B) trigonal planar
C) trigonal bipyramidal
D) octahedral
E) none of these
A) tetrahedral
B) trigonal planar
C) trigonal bipyramidal
D) octahedral
E) none of these

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
80
For the molecule below, which of the bond angles is (are) about 120  ?
?

A) C-C=O
B) H-C-C
C) C-O-H
D) O-C=O
E) both a & d
 ?
? 
A) C-C=O
B) H-C-C
C) C-O-H
D) O-C=O
E) both a & d

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck



