Deck 17: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/69
Play
Full screen (f)
Deck 17: Nuclear Chemistry
1
Indicate the missing word or words in the following definition: "Radioactive is the term used to describe nuclides that possess ________ nuclei that spontaneously emit radiation."
A) abnormally large
B) unstable
C) nonspherical
D) elongated
A) abnormally large
B) unstable
C) nonspherical
D) elongated
unstable
2
The discovery of the phenomenon of radioactivity dates back to the decade ________.
A) 1810-1820
B) 1850-1860
C) 1890-1900
D) 1930-1940
A) 1810-1820
B) 1850-1860
C) 1890-1900
D) 1930-1940
1890-1900
3
Which of the following types of radiation is composed of particles which carry a 2+ charge?
A) alpha
B) beta
C) gamma
D) positron
A) alpha
B) beta
C) gamma
D) positron
alpha
4
Which type of radioactive emission has a mass number of 4?
A) alpha particles
B) beta particles
C) gamma rays
D) positron emission
A) alpha particles
B) beta particles
C) gamma rays
D) positron emission

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
5
Which type of radioactive emission is high energy radiation without mass or charge?
A) alpha particles
B) beta particles
C) gamma rays
D) positrons
A) alpha particles
B) beta particles
C) gamma rays
D) positrons

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following nuclear changes does not represent alpha particle emission?
A)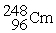
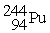
B)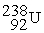
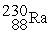
C)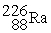
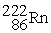
D)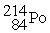
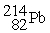
A)
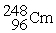
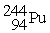
B)
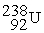
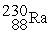
C)
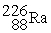
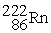
D)
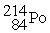
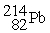

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
7
The decay of 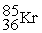 to
to 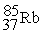 occurs through the emission of a(n) ________.
occurs through the emission of a(n) ________.
A) alpha particle
B) beta particle
C) proton
D) positron
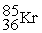 to
to 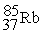 occurs through the emission of a(n) ________.
occurs through the emission of a(n) ________.A) alpha particle
B) beta particle
C) proton
D) positron

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
8
The alpha decay of 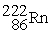 produces a nuclide of ________.
produces a nuclide of ________.
A) Po
B) U
C) La
D) Th
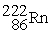 produces a nuclide of ________.
produces a nuclide of ________.A) Po
B) U
C) La
D) Th

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
9
The loss of a neutron by a radioactive nuclide causes ________.
A) no change in mass number
B) the atomic number to decrease by 4
C) the mass number to decrease
D) the atomic number to decrease by 2
A) no change in mass number
B) the atomic number to decrease by 4
C) the mass number to decrease
D) the atomic number to decrease by 2

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
10
A(n) ________ can be considered as the combination of a proton and an electron.
A) alpha particle
B) neutron
C) beta particle
D) gamma ray
A) alpha particle
B) neutron
C) beta particle
D) gamma ray

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
11
What is X in the balanced nuclear equation below?
 +
+ 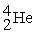 X +
X + 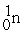
A)
B)
C)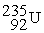
D)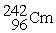
 +
+ 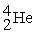 X +
X + 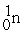
A)

B)

C)
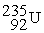
D)
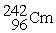

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
12
What radioactive particle is missing in the following nuclear reaction?
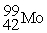
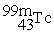 + ______
+ ______
A)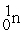
B)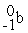
C)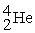
D)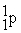
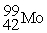
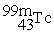 + ______
+ ______A)
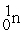
B)
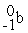
C)
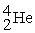
D)
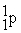

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
13
Strontium-83 has a half life of 32 hours. What fraction of a 10 mg sample of 83Sr will be left after 96 hours?
A) 50%
B) 25%
C) 12.5%
D) 6.25%
A) 50%
B) 25%
C) 12.5%
D) 6.25%

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
14
Which is an example of a beta emission reaction?
A)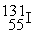
 +
+ 
B)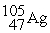 +
+ 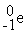
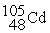
C)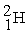 +
+ 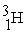
 +
+ 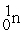
D) +
+ 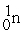
 +
+ 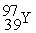 +4
+4 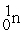
A)
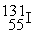
 +
+ 
B)
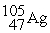 +
+ 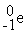
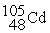
C)
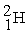 +
+ 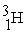
 +
+ 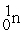
D)
 +
+ 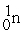
 +
+ 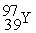 +4
+4 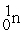

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
15
Which is an example of beta particle bombardment?
A)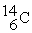
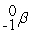 +
+ 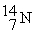
B)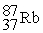 +
+ 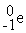
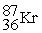
C)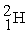 +
+ 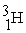
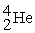 +
+ 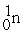
D) +
+ 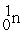
 +
+  + 4
+ 4 
A)
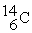
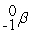 +
+ 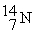
B)
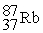 +
+ 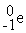
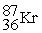
C)
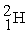 +
+ 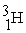
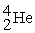 +
+ 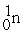
D)
 +
+ 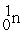
 +
+  + 4
+ 4 

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
16
Californium-251 undergoes ________ to produce curium-247.
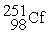
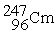 + _____
+ _____
A) gamma-ray emission
B) beta-particle decay
C) positron decay
D) alpha-particle decay
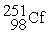
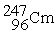 + _____
+ _____A) gamma-ray emission
B) beta-particle decay
C) positron decay
D) alpha-particle decay

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
17
After 4 half lives have elapsed, the amount of a radioactive sample which has not decayed is ________.
A) 1/4 the original amount
B) 1/8 the original amount
C) 1/16 the original amount
D) 1/2 the original amount
A) 1/4 the original amount
B) 1/8 the original amount
C) 1/16 the original amount
D) 1/2 the original amount

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
18
The amount of a radioisotope that remains after two half-lives have passed is:
A) 98%
B) 75%
C) 50%
D) 25%
A) 98%
B) 75%
C) 50%
D) 25%

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
19
The half-life of 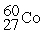 is 5.2 years. This means that after 5.2 years a sample of
is 5.2 years. This means that after 5.2 years a sample of 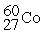 ________.
________.
A) breaks in half
B) turns into
C) has a 50% chance of exploding
D) contains one-half as many atoms as originally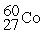
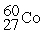 is 5.2 years. This means that after 5.2 years a sample of
is 5.2 years. This means that after 5.2 years a sample of 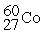 ________.
________.A) breaks in half
B) turns into

C) has a 50% chance of exploding
D) contains one-half as many atoms as originally
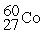

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
20
The half-life of a radionuclide is 26.4 years. The time required for a 48-gram sample of this nuclide to decay to 1.50 grams is ________.
A) 132 years
B) 106 years
C) 158 years
D) 79 years
A) 132 years
B) 106 years
C) 158 years
D) 79 years

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
21
The half-life of a radionuclide is dependent on the radionuclide's ________.
A) temperature
B) concentration
C) identity
D) state of chemical combination
A) temperature
B) concentration
C) identity
D) state of chemical combination

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
22
The half-life of carbon-14 is 5730 years. What is the approximate age of a fossil with a carbon-14 ratio to carbon 1/64 that of a living organism of the same species?
A) 28,650 years
B) 5730 years
C) 34,380 years
D) 17,190 years
A) 28,650 years
B) 5730 years
C) 34,380 years
D) 17,190 years

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
23
The half life of a radionuclide is 12.0 minutes. The time required for all except 1.25 grams of a 20.0 g sample of this nuclide to decay is ________.
A) 12.0 minutes
B) 60.0 minutes
C) 36.0 minutes
D) 48.0 minutes
A) 12.0 minutes
B) 60.0 minutes
C) 36.0 minutes
D) 48.0 minutes

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
24
Determine the half-life of a radionuclide if after 48 hours the fraction of undecayed nuclides present is 1/8.
A) 16 hours
B) 12 hours
C) 6 hours
D) 8 hours
A) 16 hours
B) 12 hours
C) 6 hours
D) 8 hours

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
25
The particles collided with target nuclei during a bombardment reaction are ________.
A) small and traveling very fast
B) small and traveling slow
C) large and traveling very fast
D) large and traveling slow
A) small and traveling very fast
B) small and traveling slow
C) large and traveling very fast
D) large and traveling slow

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
26
The bombardment of 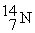 with
with 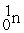 nuclei gives two products, one of which is
nuclei gives two products, one of which is 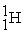 . The other product is ________.
. The other product is ________.
A)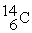
B)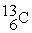
C)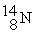
D)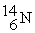
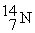 with
with 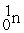 nuclei gives two products, one of which is
nuclei gives two products, one of which is 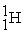 . The other product is ________.
. The other product is ________.A)
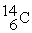
B)
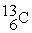
C)
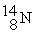
D)
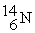

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
27
In a bombardment reaction using a neutron as the bombarding particle, the products are  nuclei and an alpha particle. Which of the following is the target nucleus for this reaction?
nuclei and an alpha particle. Which of the following is the target nucleus for this reaction?
A)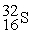
B)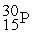
C)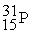
D)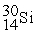
 nuclei and an alpha particle. Which of the following is the target nucleus for this reaction?
nuclei and an alpha particle. Which of the following is the target nucleus for this reaction?A)
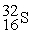
B)
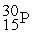
C)
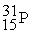
D)
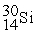

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements about synthetically produced radionuclides is incorrect?
A) Synthetic radionuclides now outnumber naturally occurring radionuclides by a 7 to 1 ratio.
B) At least one synthetic radionuclide of every naturally occurring element has been produced.
C) The mode of decay for synthetic radionuclides is always positron emission or electron capture.
D) Significant uses in the field of medicine exist for many synthetic radionuclides.
A) Synthetic radionuclides now outnumber naturally occurring radionuclides by a 7 to 1 ratio.
B) At least one synthetic radionuclide of every naturally occurring element has been produced.
C) The mode of decay for synthetic radionuclides is always positron emission or electron capture.
D) Significant uses in the field of medicine exist for many synthetic radionuclides.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
29
What is the material, X, in the nuclear bombardment process below?
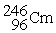 +
+ 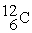 X + 4
X + 4 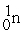
A)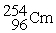
B)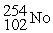
C)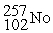
D)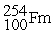
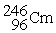 +
+ 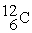 X + 4
X + 4 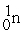
A)
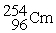
B)
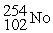
C)
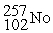
D)
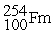

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
30
Bombardment of cadmium-113 with a(n) ________ produces cadmium-114 and a gamma ray.
A) positron
B) beta particle
C) neutron
D) alpha particle
A) positron
B) beta particle
C) neutron
D) alpha particle

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
31
The bombardment of 23Na with a 2H nuclei gives two products, one of which is hydrogen. The other product is:
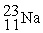 +
+ 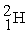
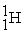 + _____
+ _____
A)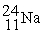
B)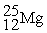
C)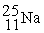
D)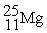
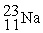 +
+ 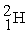
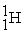 + _____
+ _____A)
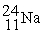
B)
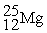
C)
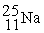
D)
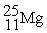

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
32
A positron is produced in a nucleus by conversion of a ________.
A) proton to a neutron
B) neutron to a proton
C) proton to an electron
D) neutron to an electron
A) proton to a neutron
B) neutron to a proton
C) proton to an electron
D) neutron to an electron

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
33
The radioactive decay of 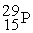 to
to 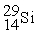 is an example of ________.
is an example of ________.
A) positron emission or electron capture
B) gamma emission or beta emission
C) alpha emission
D) beta emission
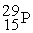 to
to 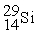 is an example of ________.
is an example of ________.A) positron emission or electron capture
B) gamma emission or beta emission
C) alpha emission
D) beta emission

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
34
Phosphorus-30 undergoes a radioactive decay process involving ________ emission to produce silicon-30.
 _____ +
_____ + 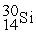
A) alpha-particle
B) positron
C) beta-particle
D) gamma-ray
 _____ +
_____ + 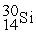
A) alpha-particle
B) positron
C) beta-particle
D) gamma-ray

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
35
Before positron emission the n/p ratio of a radionuclide is 69/55. After the emission it will be ________.
A) 70/54
B) 71/55
C) 70/56
D) 69/54
A) 70/54
B) 71/55
C) 70/56
D) 69/54

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
36
In a stable heavy nucleus the neutron to proton ratio would be approximately ________.
A) 6 to 4
B) 4 to 3
C) 3 to 1
D) 1 to 2
A) 6 to 4
B) 4 to 3
C) 3 to 1
D) 1 to 2

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
37
Radionuclides which have too low of a neutron/proton ratio generally decay through ________.
A) beta emission
B) electron capture or positron emission
C) gamma ray emission
D) alpha emission
A) beta emission
B) electron capture or positron emission
C) gamma ray emission
D) alpha emission

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the neutron-to-proton ratio after beta decay of nickel-65.
A) 2.24
B) 2.32
C) 1.24
D) 0.81
A) 2.24
B) 2.32
C) 1.24
D) 0.81

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
39
Indicate the missing words in the following statement: "A natural decay series always starts with a ________ radionuclide and ends with a ________."
A) short-lived; stable nuclide
B) short-lived; long-lived radionuclide
C) long-lived; stable nuclide
D) long-lived; short-lived radionuclide
A) short-lived; stable nuclide
B) short-lived; long-lived radionuclide
C) long-lived; stable nuclide
D) long-lived; short-lived radionuclide

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
40
In the uranium-238 decay series, the first four decay processes are alpha decay followed by two successive beta decays followed by another alpha decay. What is the intermediate product produced after these decay processes?
A) 234Pa
B) 226Ra
C) 230Th
D) 234U
A) 234Pa
B) 226Ra
C) 230Th
D) 234U

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
41
Which stable element is at the end of the uranium decay series?
A) thorium
B) uranium
C) radon
D) lead
A) thorium
B) uranium
C) radon
D) lead

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
42
Alpha and beta particles and gamma rays are called ionizing radiation because, as they travel through matter, they ________.
A) attract ions
B) repel ions
C) knock electrons off atoms or molecules in their path
D) decay into ions
A) attract ions
B) repel ions
C) knock electrons off atoms or molecules in their path
D) decay into ions

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
43
Paper acts as a shield against ________.
A) alpha particles
B) beta particles
C) gamma rays
D) both alpha particles and beta particles
A) alpha particles
B) beta particles
C) gamma rays
D) both alpha particles and beta particles

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
44
The major source of low-level radiation exposure for the "average person" is ________.
A) radon seepage
B) nuclear power production
C) fallout from weapons testing
D) medical and dental uses
A) radon seepage
B) nuclear power production
C) fallout from weapons testing
D) medical and dental uses

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
45
Which is not a criteria for selecting radionuclides for diagnostic procedures? The radionuclide must ________.
A) have a long half life
B) be detectable by instrumentation outside the body
C) have a known mechanism for elimination from the body
D) be able to be selectively transmitted to the part or system of the body that is to be studied
A) have a long half life
B) be detectable by instrumentation outside the body
C) have a known mechanism for elimination from the body
D) be able to be selectively transmitted to the part or system of the body that is to be studied

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
46
The radionuclide that serves as an external source of radiation for the treatment of cancer is ________.
A) iodine-131
B) sodium-24
C) cobalt-60
D) iron-59
A) iodine-131
B) sodium-24
C) cobalt-60
D) iron-59

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following nuclides will undergo nuclear fission when bombarded with neutrons?
A)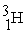
B)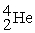
C)
D)
A)
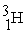
B)
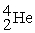
C)

D)


Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
48
In nuclear power plants, ________.
A) the fission process is used to generate steam to turn a turbine to produce electricity
B) uranium atoms are changed directly into electrical energy
C) steam is produced by passing water directly over a radioactive material
D) the principles of operation are very different from those for a coal-fired plant
A) the fission process is used to generate steam to turn a turbine to produce electricity
B) uranium atoms are changed directly into electrical energy
C) steam is produced by passing water directly over a radioactive material
D) the principles of operation are very different from those for a coal-fired plant

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is an example of natural radioactivity?
A)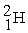 +
+ 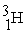
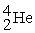 +
+ 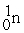
B)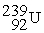
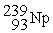 +
+ 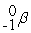
C)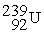 +
+ 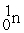
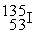 +
+ 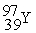 + 4
+ 4 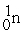
D)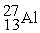 +
+ 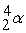
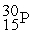 +
+ 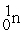
A)
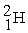 +
+ 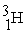
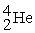 +
+ 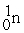
B)
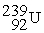
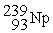 +
+ 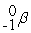
C)
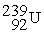 +
+ 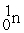
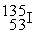 +
+ 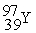 + 4
+ 4 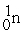
D)
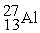 +
+ 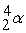
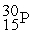 +
+ 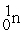

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is a fission reaction?
A)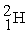 +
+ 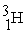
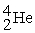 +
+ 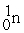
B)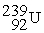
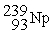 +
+ 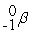
C)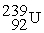 +
+ 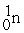
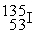 +
+ 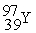 + 4
+ 4 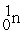
D)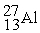 +
+ 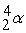
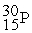 +
+ 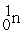
A)
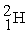 +
+ 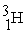
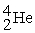 +
+ 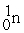
B)
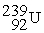
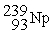 +
+ 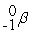
C)
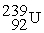 +
+ 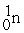
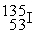 +
+ 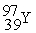 + 4
+ 4 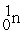
D)
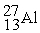 +
+ 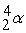
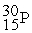 +
+ 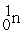

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
51
Which is an example of a fusion reaction?
A)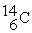
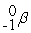 +
+ 
B)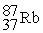 +
+ 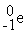
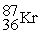
C)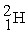 +
+ 
 +
+ 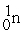
D)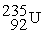 +
+ 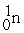
 +
+ 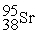 + 2
+ 2 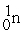
A)
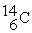
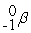 +
+ 
B)
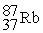 +
+ 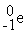
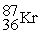
C)
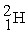 +
+ 
 +
+ 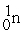
D)
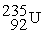 +
+ 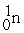
 +
+ 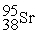 + 2
+ 2 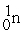

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
52
The fusion reaction that supplies the energy of the hydrogen bomb is:
A)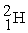 +
+ 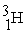
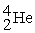 +
+ 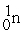
B)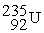 +
+ 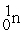
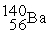 +
+ 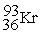 + 3
+ 3 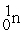
C)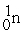 +
+ 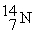
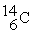 +
+ 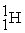
D)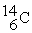
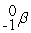 +
+ 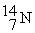
A)
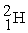 +
+ 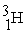
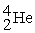 +
+ 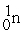
B)
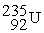 +
+ 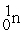
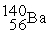 +
+ 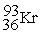 + 3
+ 3 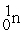
C)
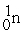 +
+ 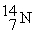
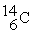 +
+ 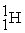
D)
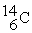
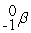 +
+ 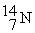

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following statements is false?
A) The reaction below is an example of a fission reaction.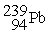 +
+ 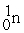
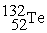 +
+ 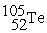 +
+ 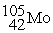 + 3
+ 3 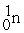
B) Neutrons are required to begin a fission process.
C) The reaction below is an example of a fusion reaction.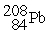 +
+ 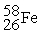
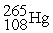 +
+ 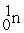
D) Extremely low temperatures are required to begin a fusion process.
A) The reaction below is an example of a fission reaction.
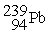 +
+ 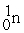
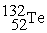 +
+ 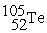 +
+ 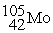 + 3
+ 3 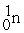
B) Neutrons are required to begin a fission process.
C) The reaction below is an example of a fusion reaction.
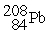 +
+ 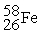
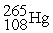 +
+ 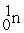
D) Extremely low temperatures are required to begin a fusion process.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
54
Which response contains all of the following that are characteristics of nuclear fusion, and no others?
I. Large quantities of energy are released in the process.
II. The process is used to produce some electricity in the United States.
III. Transmutation of elements occurs.
IV. An example of this process occurs on the Sun.
V. Neutrons are required to initiate the process.
A) I, III, and IV
B) I and IV
C) II, III, and V
D) I, II, and IV
I. Large quantities of energy are released in the process.
II. The process is used to produce some electricity in the United States.
III. Transmutation of elements occurs.
IV. An example of this process occurs on the Sun.
V. Neutrons are required to initiate the process.
A) I, III, and IV
B) I and IV
C) II, III, and V
D) I, II, and IV

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
55
Nuclear processes differ from chemical processes in that nuclear processes ________.
A) are never spontaneous
B) produce smaller amounts of energy
C) cause some elements to change their identities
D) always require 2 reactants
A) are never spontaneous
B) produce smaller amounts of energy
C) cause some elements to change their identities
D) always require 2 reactants

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
56
Write a balanced nuclear equation for the alpha-particle decay of a 138Xe isotope.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
57
Write a balanced nuclear equation for the beta-particle decay of a 211Bi isotope.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
58
Write a nuclear equation for the bombardment of a radionuclide with an alpha-particle which produces curium-242 and one neutron.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
59
Complete the following nuclear reactions:
-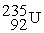 →
→ 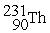 + ________
+ ________
-
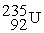 →
→ 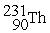 + ________
+ ________
Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
60
Complete the following nuclear reactions:
-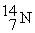 +
+ 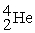 → ________ +
→ ________ + 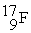
-
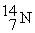 +
+ 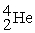 → ________ +
→ ________ + 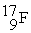

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
61
Complete the following nuclear reactions:
-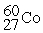 →
→ 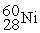 + ________
+ ________
-
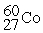 →
→ 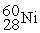 + ________
+ ________
Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
62
Complete the following nuclear reactions:
-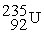 +
+ 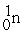 →________ +
→________ + 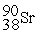 + 3
+ 3 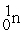
-
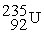 +
+ 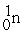 →________ +
→________ + 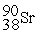 + 3
+ 3 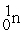

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
63
Complete the following nuclear reactions:
-________ →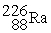 +
+ 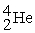
-________ →
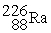 +
+ 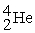

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
64
What is the intermediate product produced after the first three decays of 223Fr?
233Fr → beta → alpha → alpha → ?
233Fr → beta → alpha → alpha → ?

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
65
Write a nuclear equation for the following processes:
-Bismuth-214 undergoes beta decay
-Bismuth-214 undergoes beta decay

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
66
Write a nuclear equation for the following processes:
-Thorium-230 decays to the radium-226 isotope
-Thorium-230 decays to the radium-226 isotope

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
67
Indicate whether each of the following is a fission reaction, a fusion reaction, or neither a fission or fusion reaction.
-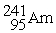 +
+ 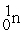
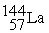 +
+ 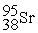 + 3
+ 3 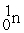 ____________________
____________________
A) fission
B) neither
C) fusion
-
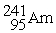 +
+ 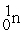
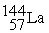 +
+ 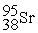 + 3
+ 3 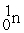 ____________________
____________________A) fission
B) neither
C) fusion

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
68
Indicate whether each of the following is a fission reaction, a fusion reaction, or neither a fission or fusion reaction.
-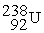
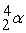 +
+ 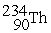 ____________________
____________________
A) fission
B) neither
C) fusion
-
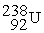
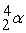 +
+ 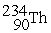 ____________________
____________________A) fission
B) neither
C) fusion

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
69
Indicate whether each of the following is a fission reaction, a fusion reaction, or neither a fission or fusion reaction.
-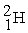 +
+ 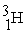
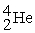 +
+ 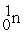 ____________________
____________________
A) fission
B) neither
C) fusion
-
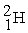 +
+ 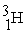
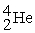 +
+ 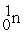 ____________________
____________________A) fission
B) neither
C) fusion

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck



