Deck 9: Chemical Calculations: the Mole Concept and Chemical Formulas
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/93
Play
Full screen (f)
Deck 9: Chemical Calculations: the Mole Concept and Chemical Formulas
1
Which of the following information items would be most useful in verifying the law of definite proportions?
A) analysis information about two compounds that contain the same elements in different proportions
B) a number of samples of a pure compound collected from various locations
C) the total mass of the reactants in a series of reactions
D) the total mass of the products in a series of reactions
A) analysis information about two compounds that contain the same elements in different proportions
B) a number of samples of a pure compound collected from various locations
C) the total mass of the reactants in a series of reactions
D) the total mass of the products in a series of reactions
a number of samples of a pure compound collected from various locations
2
According to the law of definite proportions, if a sample of a compound contains 24.00 grams of carbon and 4.00 grams of hydrogen, then another sample of the same compound which contains 48.0 grams of carbon must contain ________.
A) 16.0 g of hydrogen
B) 12.0 g of hydrogen
C) 24.0 g of hydrogen
D) 8.00 g of hydrogen
A) 16.0 g of hydrogen
B) 12.0 g of hydrogen
C) 24.0 g of hydrogen
D) 8.00 g of hydrogen
8.00 g of hydrogen
3
What is the formula weight of Ba(C2H3O2)2 in grams per mole?
A) 392.71
B) 255.43
C) 196.38
D) 306.42
A) 392.71
B) 255.43
C) 196.38
D) 306.42
255.43
4
Which of the following compounds has the largest formula mass?
A) BF3
B) NO2
C) HCN
D) CCl4
A) BF3
B) NO2
C) HCN
D) CCl4

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
5
The formula mass of (NH4)3PO4, is ________.
A) 121.12 amu
B) 149.12 amu
C) 110.12 amu
D) 159.68 amu
A) 121.12 amu
B) 149.12 amu
C) 110.12 amu
D) 159.68 amu

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
6
Glucose is the type of sugar the human body metabolizes to produce energy. Calculate the formula mass of glucose, C6H12O6.
A) 180.18 amu
B) 220.02 amu
C) 168.18 amu
D) 340.00 amu
A) 180.18 amu
B) 220.02 amu
C) 168.18 amu
D) 340.00 amu

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
7
What is the molar mass of crotonaldehyde (C4H6O)?
A) 54.1 g/mol
B) 70.1 g/mol
C) 64.0 g/mol
D) 70.0 g/mol
A) 54.1 g/mol
B) 70.1 g/mol
C) 64.0 g/mol
D) 70.0 g/mol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
8
The mass percentage of Cl in the compound KClO3 is ________.
A) 53.87%
B) 64.19%
C) 33.33%
D) 28.93%
A) 53.87%
B) 64.19%
C) 33.33%
D) 28.93%

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
9
In which of the following compounds is the mass percentage of calcium the greatest?
A) Ca(C2H3O2)2
B) CaCl2
C) CaH2
D) Ca(NO3)2
A) Ca(C2H3O2)2
B) CaCl2
C) CaH2
D) Ca(NO3)2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
10
In which of the following samples will the mass percentage H be the greatest?
A) 3.25 g of C2H6
B) 15.0 g of C2H6
C) 36.0 g of C2H6
D) All samples have the same mass percentage H.
A) 3.25 g of C2H6
B) 15.0 g of C2H6
C) 36.0 g of C2H6
D) All samples have the same mass percentage H.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
11
A 2.00 g sample of cholesterol contains 1.68 g C, 0.24 g H, and 0.080 g O. What is the percent composition of oxygen in cholesterol?
A) 4.0%
B) 76%
C) 91%
D) 56%
A) 4.0%
B) 76%
C) 91%
D) 56%

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
12
Calculate the percentage composition of oxygen in Ca(C2H3O2)2.
A) 40.47%
B) 40.46%
C) 29.74%
D) 46.39%
A) 40.47%
B) 40.46%
C) 29.74%
D) 46.39%

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
13
The mass percent of chlorine in Mg(ClO3)2 is ________.
A) 33.0%
B) 37.1%
C) 43.6%
D) 12.7%
A) 33.0%
B) 37.1%
C) 43.6%
D) 12.7%

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
14
What is the mass percent of carbon in dimethyl sulfoxide, C2H6SO?
A) 20.6%
B) 60.0%
C) 30.7%
D) 79.8%
A) 20.6%
B) 60.0%
C) 30.7%
D) 79.8%

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the percentage composition of oxygen in K2CO3.
A) 34.73%
B) 11.65%
C) 13.39%
D) 39.82%
A) 34.73%
B) 11.65%
C) 13.39%
D) 39.82%

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
16
Nicotine (C10H14N2) is a byproduct of the tobacco industry and is used as an agricultural insecticide. Calculate the percent composition of each element in nicotine.
A) 74.0 % C 8.70 % H 17.3 % N
B) 83.7 % C 4.45 % H 11.9 % N
C) 70.2 % C 10.5 % H 10.3 % N
D) 90.0 % C 1.50 % H 8.50 % N
A) 74.0 % C 8.70 % H 17.3 % N
B) 83.7 % C 4.45 % H 11.9 % N
C) 70.2 % C 10.5 % H 10.3 % N
D) 90.0 % C 1.50 % H 8.50 % N

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements concerning the numerical value 6.02 x 1023 is incorrect?
A) It is called Avogadro's number.
B) It is the number of carbon atoms in one mole of CO2 molecules.
C) It is the mass, in grams, of one mole of any substance.
D) It is the number of atoms in one mole of He atoms.
A) It is called Avogadro's number.
B) It is the number of carbon atoms in one mole of CO2 molecules.
C) It is the mass, in grams, of one mole of any substance.
D) It is the number of atoms in one mole of He atoms.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following quantities does not contain the same number of atoms as there are in 80.2 grams of Ca?
A) 0.50 moles of P4 molecules
B) 64.2 grams of S
C) 6.02 x 1023 molecules of O2
D) 6.02 x 1023 atoms of Ni
A) 0.50 moles of P4 molecules
B) 64.2 grams of S
C) 6.02 x 1023 molecules of O2
D) 6.02 x 1023 atoms of Ni

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
19
How many Cl atoms are present in 2.16 moles of NCl3?
A) 2.4 x 1022 Cl atoms
B) 8.4 x 1024 Cl atoms
C) 3.90 x 1024 Cl atoms
D) 6.6 x 10-26 Cl atoms
A) 2.4 x 1022 Cl atoms
B) 8.4 x 1024 Cl atoms
C) 3.90 x 1024 Cl atoms
D) 6.6 x 10-26 Cl atoms

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
20
Calculate the mass (grams) of 0.750 moles of Al2(Cr2O7)3.
A) 526 g
B) 702.0 g
C) 270.0 g
D) 486.0 g
A) 526 g
B) 702.0 g
C) 270.0 g
D) 486.0 g

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the number of iron atoms in 6.98 x 10-3 grams of iron.
A) 9.37 x 1028 atoms
B) 3.92 x 1019 atoms
C) 3.24 x 1023 atoms
D) 7.53 x 1019 atoms
A) 9.37 x 1028 atoms
B) 3.92 x 1019 atoms
C) 3.24 x 1023 atoms
D) 7.53 x 1019 atoms

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements is correct concerning common table sugar, C12H22O11?
A) The total number of hydrogen atoms present in one molecule is 22.
B) The total number of carbon atoms present in three moles of sugar is equal to 36 (6.022 x 1023).
C) The mass of 0.600 moles of sugar is 205 grams.
D) All of the above statements are correct.
A) The total number of hydrogen atoms present in one molecule is 22.
B) The total number of carbon atoms present in three moles of sugar is equal to 36 (6.022 x 1023).
C) The mass of 0.600 moles of sugar is 205 grams.
D) All of the above statements are correct.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the number of lithium atoms in 3.66 moles of lithium.
A) 2.20 x 1024
B) 8.97 x 1026
C) 3.54 x 1025
D) 6.23 x 1025
A) 2.20 x 1024
B) 8.97 x 1026
C) 3.54 x 1025
D) 6.23 x 1025

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
24
Acetic acid (C2H4O2) is the principle component of vinegar. 96.0 g of C2H4O2 is equivalent to ________ moles of C2H4O2 and contains ________ hydrogen (H) atoms.
A) 1.60; 9.64 x 1023
B) 0.626; 3.85 x 1024
C) 0.943; 7.29 x 1024
D) 1.60; 3.85 x 1024
A) 1.60; 9.64 x 1023
B) 0.626; 3.85 x 1024
C) 0.943; 7.29 x 1024
D) 1.60; 3.85 x 1024

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
25
You have been given 3.50 moles of ammonia, NH3. How many molecules of ammonia does it contain?
A) 4.75 x 10-25
B) 2.11 x 1024
C) 1.81 x 1024
D) 2.21 x 10-24
A) 4.75 x 10-25
B) 2.11 x 1024
C) 1.81 x 1024
D) 2.21 x 10-24

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
26
How many molecules of ethanol, C2H5OH, are contained in 150. gram sample?
A) 46.0
B) 6.02 x 1023
C) 1.96 x 1024
D) 5.1 x 10-25
A) 46.0
B) 6.02 x 1023
C) 1.96 x 1024
D) 5.1 x 10-25

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
27
Sugar has an empirical formula of CH2O and a molar mass of 180.2 g/mol. If one teaspoon of sugar weighs 3.50 grams, how many moles and molecules of sugar are present?
A) 0.117 moles, 7.03 x 1022 molecules
B) 0.0194 moles, 3.24 x 1026 molecules
C) 0.0194 moles, 1.17 x 1022 molecules
D) 0.0583 moles, 3.51 x 1022 molecules
A) 0.117 moles, 7.03 x 1022 molecules
B) 0.0194 moles, 3.24 x 1026 molecules
C) 0.0194 moles, 1.17 x 1022 molecules
D) 0.0583 moles, 3.51 x 1022 molecules

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements is incorrect concerning BaF2?
A) The total number of fluorine ions present in one formula unit is 2 (6.02 x 1023).
B) The total number of fluoride ions present in three moles of BaF2 is equal to 6 (6.02 x 1023).
C) The mass of 0.600 moles of BaF2 is 105 grams.
D) 0.600 moles of BaF2 is equivalent to 3.61 x 1023 formula units of BaF2.
A) The total number of fluorine ions present in one formula unit is 2 (6.02 x 1023).
B) The total number of fluoride ions present in three moles of BaF2 is equal to 6 (6.02 x 1023).
C) The mass of 0.600 moles of BaF2 is 105 grams.
D) 0.600 moles of BaF2 is equivalent to 3.61 x 1023 formula units of BaF2.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
29
Avogadro's number of aluminum atoms has a mass equal to ________.
A) 6.02 x 1023 g of aluminum
B) 14.01 g of nitrogen
C) 26.98 g of aluminum
D) 6.02 x 10-23 g of calcium
A) 6.02 x 1023 g of aluminum
B) 14.01 g of nitrogen
C) 26.98 g of aluminum
D) 6.02 x 10-23 g of calcium

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
30
Which of these samples contains the largest number of particles?
A) 0.20 g of H2 molecules
B) 0.10 mol of N2 molecules
C) 1.90 g of F atoms
D) 0.20 mol of He atoms
A) 0.20 g of H2 molecules
B) 0.10 mol of N2 molecules
C) 1.90 g of F atoms
D) 0.20 mol of He atoms

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements about "one-half mole of H2O" is incorrect?
A) It contains the same number of molecules as one-half mole of CCl4.
B) It has the same mass, in grams, as one-half mole of CCl4.
C) It contains 3.01 x 1023 molecules.
D) It has a mass of 9.0 grams.
A) It contains the same number of molecules as one-half mole of CCl4.
B) It has the same mass, in grams, as one-half mole of CCl4.
C) It contains 3.01 x 1023 molecules.
D) It has a mass of 9.0 grams.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following contains the greatest number of atoms?
A) 0.25 mole SO2
B) 0.75 moles He
C) 0.50 moles Cl2O
D) 0.70 moles NH3
A) 0.25 mole SO2
B) 0.75 moles He
C) 0.50 moles Cl2O
D) 0.70 moles NH3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
33
The number of atoms contained in 16.0 grams of Mg would be the same as the number of molecules contained in ________ grams of N2O.
A) 57.6
B) 29.0
C) 38.0
D) 66.2
A) 57.6
B) 29.0
C) 38.0
D) 66.2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
34
An atom of an element has a mass of 1.0555 x 10-22 grams. What is the atomic mass of the element?
A) 63.541 amu
B) 196.90 amu
C) 179.20 amu
D) 195.11 amu
A) 63.541 amu
B) 196.90 amu
C) 179.20 amu
D) 195.11 amu

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
35
How many grams are present in 6.5 moles of Cu(NO3)2?
A) 124 g
B) 404 g
C) 1080 g
D) 1200 g
A) 124 g
B) 404 g
C) 1080 g
D) 1200 g

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
36
Which element contains atoms with an average mass of 1.79 x 10-22 grams?
A) Fe
B) Sc
C) Ag
D) F
A) Fe
B) Sc
C) Ag
D) F

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
37
What is the molar mass of a substance if 0.5602 moles of the substance has a mass of 19.72 grams?
A) 24.30
B) 35.20
C) 99.00
D) 101.0
A) 24.30
B) 35.20
C) 99.00
D) 101.0

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
38
A beaker contains 0.964 grams of nitrogen. How many moles of molecular nitrogen does the beaker contain?
A) 0.0688 mol
B) 2.262 x 1023 mol
C) 0.0344 mol
D) 2.64 mol
A) 0.0688 mol
B) 2.262 x 1023 mol
C) 0.0344 mol
D) 2.64 mol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the mass of 1.98 moles of Li2SO3.
A) 186
B) 155.37
C) 96.13
D) 316.40
A) 186
B) 155.37
C) 96.13
D) 316.40

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
40
One mole of OF2 molecules contains ________.
A) 16.0 atoms of O
B) 38.0 atoms of F
C) 2 moles of F atoms
D) one O atom
A) 16.0 atoms of O
B) 38.0 atoms of F
C) 2 moles of F atoms
D) one O atom

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
41
2.00 formula units of the compound K2S contains ________.
A) 4 K atoms
B) 2.00 moles of S atoms
C) 2.00 grams of K2S
D) 39.09 g of K
A) 4 K atoms
B) 2.00 moles of S atoms
C) 2.00 grams of K2S
D) 39.09 g of K

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the number of moles of aspirin, C9H8O4, in a 4.0 gram tablet.
A) 2.2 x 10-4
B) 2.2
C) 4.6 x 10-3
D) 0.022
A) 2.2 x 10-4
B) 2.2
C) 4.6 x 10-3
D) 0.022

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
43
How many moles of nitrogen, N2, are contained in a 35.0 g sample?
A) 1.25 moles
B) 0.80 moles
C) 28.0 mol
D) 0.50 mol
A) 1.25 moles
B) 0.80 moles
C) 28.0 mol
D) 0.50 mol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
44
How many grams of carbon are present in 26.2 grams of H2CO3?
A) 11.60 g
B) 16.8 g
C) 47.3 g
D) 5.07 g
A) 11.60 g
B) 16.8 g
C) 47.3 g
D) 5.07 g

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is the correct "set-up" for the problem "How many atoms are present in 15.0 g Na?"
A) 15.0 g Na ×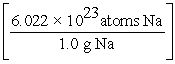
B) 15.0 g Na × ×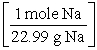
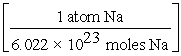
C) 15.0 g Na × ×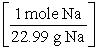
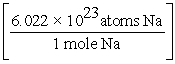
D) 15.0 g Na ×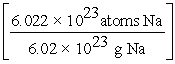
A) 15.0 g Na ×
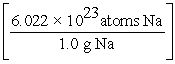
B) 15.0 g Na × ×
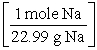
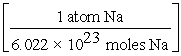
C) 15.0 g Na × ×
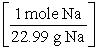
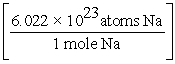
D) 15.0 g Na ×
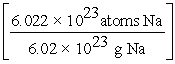

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
46
The "set-up" for the problem "What is the mass, in grams, of 2.50 × 1022 atoms of Ca?" below is correct, except numbers in the last conversion factor have been replaced by the letters A and B. What are the numerical values of A and B, respectively?
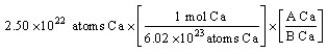
A) 1 mole and 6.02 x 1023 atoms
B) 40.08 g and 1 mol
C) 6.02 x 1023 g and 1 atom
D) 40.08 mol and 6.02 x 1023 atoms
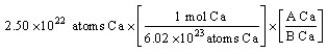
A) 1 mole and 6.02 x 1023 atoms
B) 40.08 g and 1 mol
C) 6.02 x 1023 g and 1 atom
D) 40.08 mol and 6.02 x 1023 atoms

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the correct "set-up" for the problem "How many moles of C are present in 25.00 grams of C6H12O6?"
A)
B)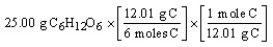
C)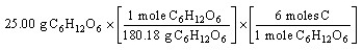
D)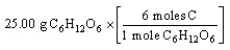
A)

B)
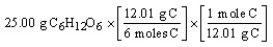
C)
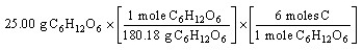
D)
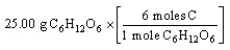

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
48
The "set-up" for the problem "How many oxygen atoms are there in 4.0 moles of N2O4?" below is correct, except for the use of letters rather than numbers in the conversion factors. What are the correct numerical values of A and D, respectively?
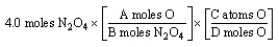
A) 4 and 4
B) 1 and 4
C) 4 and 1
D) 4 and 6.02 1023
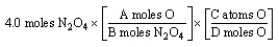
A) 4 and 4
B) 1 and 4
C) 4 and 1
D) 4 and 6.02 1023

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is the correct "set-up" for the problem "How many grams of S are present in 50.0 g of S4N4?"
A)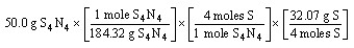
B)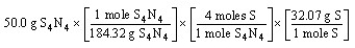
C)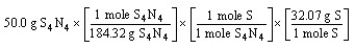
D)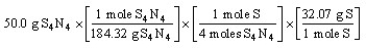
A)
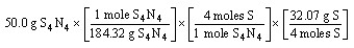
B)
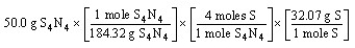
C)
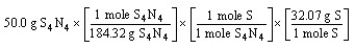
D)
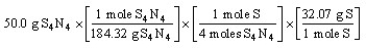

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
50
The number of moles of O atoms in 27 g of OF2 is ________.
A) 13 moles
B) 6.4 moles
C) 0.50 moles
D) 0.20 moles
A) 13 moles
B) 6.4 moles
C) 0.50 moles
D) 0.20 moles

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
51
How many atoms of H are present in 27.6 g of NH3?
A) 2.93 x 1024
B) 3.67 x 1023
C) 9.79 x 1024
D) 3.55 x 1026
A) 2.93 x 1024
B) 3.67 x 1023
C) 9.79 x 1024
D) 3.55 x 1026

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
52
How many atoms are present in 0.65 moles of P4O10?
A) 8.71 x 1024 atoms
B) 2.49 x 1024 atoms
C) 2.49 x 1025 atoms
D) 5.5 x 1024 atoms
A) 8.71 x 1024 atoms
B) 2.49 x 1024 atoms
C) 2.49 x 1025 atoms
D) 5.5 x 1024 atoms

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
53
Calculate the number of potassium ions present in a 16.42 gram sample of K3P.
A) 2.931 x 1024 potassium ions
B) 2.004 x 1023 potassium ions
C) 1.466 x 1024 potassium ions
D) 2.474 x 1023 potassium ions
A) 2.931 x 1024 potassium ions
B) 2.004 x 1023 potassium ions
C) 1.466 x 1024 potassium ions
D) 2.474 x 1023 potassium ions

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
54
How many carbon atoms are there in 25.0 grams of Al2(CO3)3?
A) 5.96 x 1024 atoms
B) 4.23 x 1022 atoms
C) 7.08 x 1025 atoms
D) 1.93 x 1023 atoms
A) 5.96 x 1024 atoms
B) 4.23 x 1022 atoms
C) 7.08 x 1025 atoms
D) 1.93 x 1023 atoms

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is the empirical formula for the compound C2H4O2?
A) C3H6O3
B) CH2O
C) CHO
D) C2HO
A) C3H6O3
B) CH2O
C) CHO
D) C2HO

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
56
The empirical formula of C10H20 is ________.
A) C10H20
B) C5H10
C) C2H4
D) CH2
A) C10H20
B) C5H10
C) C2H4
D) CH2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
57
The empirical formula of a compound is CH2O and its formula weight is 120.12 amu. What is the molecular formula?
A) C2H4O2
B) C3H6O3
C) C4H8O4
D) C5H10O5
A) C2H4O2
B) C3H6O3
C) C4H8O4
D) C5H10O5

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
58
What is the empirical formula of a compound that contains 78.11% B and 21.89% H?
A) BH2
B) BH3
C) B2H6
D) B2H3
A) BH2
B) BH3
C) B2H6
D) B2H3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
59
What is the molecular formula of a compound that contains 40.0% C, 6.71 % H, and 53.29% O? The molecular mass of this compound is 60.05.
A) C2H4O2
B) CH2O
C) C2H3O4
D) C2H2O4
A) C2H4O2
B) CH2O
C) C2H3O4
D) C2H2O4

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
60
Analysis of a sample of a compound shows that 0.130 mole of Cl, 0.130 mole of H and 0.520 mole of O are present. What is the empirical formula of the compound?
A) HClO
B) HClO2
C) HClO3
D) HClO4
A) HClO
B) HClO2
C) HClO3
D) HClO4

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
61
If a 1.59 gram sample of a carbon-containing compound is burned in air, 1.01 gram of CO2 is produced. What is the percent carbon in the compound?
A) 29%
B) 17%
C) 55%
D) 92%
A) 29%
B) 17%
C) 55%
D) 92%

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
62
Analysis of an unknown nickel oxide compound indicated that the molar ratio of nickel to oxygen was 0.167 to 0.251, respectively. What is the empirical formula for the compound?
A) Ni3O4
B) NiO
C) NiO3
D) Ni2O3
A) Ni3O4
B) NiO
C) NiO3
D) Ni2O3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
63
A sample of an ionic compound was analyzed and found to contain 6.072 g of sodium, 8.474 g of sulfur, and 6.336 g of oxygen. What is its empirical formula?
A) NaSO3
B) Na2SO3
C) Na2S2O3
D) Na2SO4
A) NaSO3
B) Na2SO3
C) Na2S2O3
D) Na2SO4

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
64
Analysis of an unknown sample indicated the sample contained 0.140 grams of N and 0.320 grams of O. The molar mass of the compound was determined to be 92.02 amu. What is the molecular formula of the compound?
A) NO
B) N2O
C) N2O4
D) NO2
A) NO
B) N2O
C) N2O4
D) NO2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
65
Carbohydrates have an empirical formula of CH2O. What is the molecular formula of the sugar maltose if it has a molar mass of 180.18?
A) C3H6O3
B) C4H8O4
C) C5H10O5
D) C6H12O6
A) C3H6O3
B) C4H8O4
C) C5H10O5
D) C6H12O6

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
66
Octane is a principle component of gasoline. The empirical formula of octane is C4H9, and the molar mass of octane is 114.26 grams per mole. What is the molecular formula of octane?
A) C4H9
B) C12H36
C) C12H27
D) C8H18
A) C4H9
B) C12H36
C) C12H27
D) C8H18

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
67
The empirical formula of a compound was determined to be P2O3. The molecular weight of the compound is 220 g/mol. What is the molecular formula of the compound?
A) P2O3
B) P4O6
C) P2O5
D) P4O10
A) P2O3
B) P4O6
C) P2O5
D) P4O10

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
68
Determine the percentage composition for the compound C10H14N2.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
69
Match the statements on the left with an appropriate response from the right. Responses on the right may be used more than once or need not be used at all.
-________ has a mass of 4.0 grams
A) 1/4 mole CH4
B) 1/4 mole NH3
C) 2 moles H2O
D) 1/2 mole N2H
-________ has a mass of 4.0 grams
A) 1/4 mole CH4
B) 1/4 mole NH3
C) 2 moles H2O
D) 1/2 mole N2H

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
70
Match the statements on the left with an appropriate response from the right. Responses on the right may be used more than once or need not be used at all.
-________ contains 1.20 x 1024 molecules
A) 1/4 mole CH4
B) 1/4 mole NH3
C) 2 moles H2O
D) 1/2 mole N2H
-________ contains 1.20 x 1024 molecules
A) 1/4 mole CH4
B) 1/4 mole NH3
C) 2 moles H2O
D) 1/2 mole N2H

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
71
Match the statements on the left with an appropriate response from the right. Responses on the right may be used more than once or need not be used at all.
-________ contains 1 mole of atoms
A) 1/4 mole CH4
B) 1/4 mole NH3
C) 2 moles H2O
D) 1/2 mole N2H
-________ contains 1 mole of atoms
A) 1/4 mole CH4
B) 1/4 mole NH3
C) 2 moles H2O
D) 1/2 mole N2H

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
72
Match the statements on the left with an appropriate response from the right. Responses on the right may be used more than once or need not be used at all.
-________ contains 4 moles of H atoms
A) 1/4 mole CH4
B) 1/4 mole NH3
C) 2 moles H2O
D) 1/2 mole N2H
-________ contains 4 moles of H atoms
A) 1/4 mole CH4
B) 1/4 mole NH3
C) 2 moles H2O
D) 1/2 mole N2H

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
73
Contrast the two members of each pair in the statements on the left, and then select your response from the response list on the right.
-________(I) formula mass of CBr4(II) formula mass of P2S3
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.
-________(I) formula mass of CBr4(II) formula mass of P2S3
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
74
Contrast the two members of each pair in the statements on the left, and then select your response from the response list on the right.
-________(I) grams in 1 mole of SiF4(II) grams in 1 mole of AsF3
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.
-________(I) grams in 1 mole of SiF4(II) grams in 1 mole of AsF3
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
75
Contrast the two members of each pair in the statements on the left, and then select your response from the response list on the right.
-________(I) moles in 50 grams of N2(II) moles in 50 grams of H2S
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.
-________(I) moles in 50 grams of N2(II) moles in 50 grams of H2S
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
76
Contrast the two members of each pair in the statements on the left, and then select your response from the response list on the right.
-________(I) molecules in 3 moles of SO2(II) molecules in 3 moles of SO3
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.
-________(I) molecules in 3 moles of SO2(II) molecules in 3 moles of SO3
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
77
Contrast the two members of each pair in the statements on the left, and then select your response from the response list on the right.
-________(I) atoms in 2 moles of NO2(II) atoms in 2 moles of N2O
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.
-________(I) atoms in 2 moles of NO2(II) atoms in 2 moles of N2O
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
78
Contrast the two members of each pair in the statements on the left, and then select your response from the response list on the right.
-________(I) molecules in 28 grams of Cl2(II) molecules in 28 grams of NaCl
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.
-________(I) molecules in 28 grams of Cl2(II) molecules in 28 grams of NaCl
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
79
Contrast the two members of each pair in the statements on the left, and then select your response from the response list on the right.
-________(I) moles of N atoms in 34 g NH3(II) moles of N atoms in 28 g of N2
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.
-________(I) moles of N atoms in 34 g NH3(II) moles of N atoms in 28 g of N2
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
80
Contrast the two members of each pair in the statements on the left, and then select your response from the response list on the right.
-________(I) moles of O atoms in Avogadro's number of SO2 molecules(II) moles of O atoms in twice Avogadro's number of CO molecule
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.
-________(I) moles of O atoms in Avogadro's number of SO2 molecules(II) moles of O atoms in twice Avogadro's number of CO molecule
A) I is greater than II.
B) I is less than II.
C) I is equal to II.
D) It is impossible to make a comparison between I and II.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck



