Deck 7: Chemical Bonds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 7: Chemical Bonds
1
How many valence electrons does the representative element with the electron configuration 1s2 2s2 2p6 3s2 3p5 possess?
A) 5
B) 3
C) 6
D) 7
A) 5
B) 3
C) 6
D) 7
7
2
Elements in groups IIA and VA of the periodic table possess, respectively, how many valence electrons?
A) 2 and 2
B) 2 and 6
C) 3 and 4
D) 2 and 5
A) 2 and 2
B) 2 and 6
C) 3 and 4
D) 2 and 5
2 and 5
3
Which of the following is the correct Lewis symbol diagram for carbon?
A)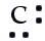
B)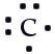
C)
D)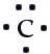
A)
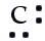
B)
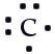
C)

D)
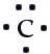

4
Determine the number of valence electrons in an atom of antimony.
A) 1
B) 2
C) 4
D) 5
A) 1
B) 2
C) 4
D) 5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about the noble gases is incorrect?
A) All exist in nature as individual atoms rather than molecular form.
B) They are the most reactive of all gases.
C) All have very stable electron arrangements.
D) All have 8 valence electrons.
A) All exist in nature as individual atoms rather than molecular form.
B) They are the most reactive of all gases.
C) All have very stable electron arrangements.
D) All have 8 valence electrons.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
Which option will correctly fill in the blank to define the octet rule? Atoms will ________ electrons so each atom involved will have a noble gas configuration.
A) share
B) lose
C) lose or gain
D) lose, gain, or share
A) share
B) lose
C) lose or gain
D) lose, gain, or share

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
The notation Y3- denotes a Y atom that has ________.
A) lost 3 electrons
B) lost 3 protons
C) gained 3 electrons
D) gained 3 protons
A) lost 3 electrons
B) lost 3 protons
C) gained 3 electrons
D) gained 3 protons

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following ions would not possess an "octet of electrons?"
A) P2-
B) Be2+
C) K+
D) S2-
A) P2-
B) Be2+
C) K+
D) S2-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
Which statement is incorrect?
A) An anion is a negatively charged ion.
B) Ions have different chemical properties than its corresponding atom.
C) Ionic bond is the attractive force between positively and negatively charged ions.
D) Ions are electrically neutral.
A) An anion is a negatively charged ion.
B) Ions have different chemical properties than its corresponding atom.
C) Ionic bond is the attractive force between positively and negatively charged ions.
D) Ions are electrically neutral.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
Which is the electron configuration for a Sc3+ ion?
A) 1s2 2s2 2p6 3s2 3p6
B) 1s2 2s2 2p6
C) 1s2 2s2 2p 3s2
D) 1s2 2s2 2p6 3s2 3p3
A) 1s2 2s2 2p6 3s2 3p6
B) 1s2 2s2 2p6
C) 1s2 2s2 2p 3s2
D) 1s2 2s2 2p6 3s2 3p3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following represents the electron configuration of a selenium ion in its common oxidation state?
A) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
B) 1s2 2s2 2p6 3s2 3p6 4s2 3d10
C) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
D) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
A) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
B) 1s2 2s2 2p6 3s2 3p6 4s2 3d10
C) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
D) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
Elements in groups IIA and VIA of the periodic table would, respectively, be expected to form ions with charges of ________.
A) +1 and +7
B) -1 and -5
C) +1 and -1
D) +2 and-2
A) +1 and +7
B) -1 and -5
C) +1 and -1
D) +2 and-2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
The electron configuration for a Al3+ ion is ________.
A) 1s2 2s2 2p6
B) 1s2 2s2 2p6 3s2 3p1
C) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4 p6
D) 1s2 2s2 2p6 3s2 3p6 4s2
A) 1s2 2s2 2p6
B) 1s2 2s2 2p6 3s2 3p1
C) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4 p6
D) 1s2 2s2 2p6 3s2 3p6 4s2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
The electron configuration 1s2 2s2 2p6 3s2 3p6 fits all of the following species except one. The exception is ________.
A) F-
B) Ca2+
C) S2-
D) P3-
A) F-
B) Ca2+
C) S2-
D) P3-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following compounds contains an ion with a 3+ charge?
A) KCl
B) AlP
C) BeF2
D) BaO
A) KCl
B) AlP
C) BeF2
D) BaO

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
What behavior is expected from an atom with the electronic configuration of

A) gain of 2 electrons
B) loss of 2 electrons
C) gain of 4 electrons
D) loss of 4 electrons

A) gain of 2 electrons
B) loss of 2 electrons
C) gain of 4 electrons
D) loss of 4 electrons

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
Which ion is isoelectronic with Kr?
A) Cl-
B) O2-
C) Rb+
D) Ba2+
A) Cl-
B) O2-
C) Rb+
D) Ba2+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is not isoelectronic with a noble gas?
A) Li+
B) Ba2+
C) S-
D) F-
A) Li+
B) Ba2+
C) S-
D) F-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following sets contains species that are all isoelectronic?
A) O, F, Ne
B) C+4, N-3, O-2
C) P-3, S-2, Ar
D) Na, Mg, Al
A) O, F, Ne
B) C+4, N-3, O-2
C) P-3, S-2, Ar
D) Na, Mg, Al

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds contains an ion with a 2- charge?
A) AlP
B) MgS
C) KBr
D) BaCl2
A) AlP
B) MgS
C) KBr
D) BaCl2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
In the formation of the ionic compound CaBr2 the number of electrons transferred from one Ca atom to two Br atoms, per formula unit, is ________.
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
In the process of forming sodium nitride, Na3N, each sodium atom ________ electron(s) and each nitride atom ________ electron(s).
A) loses one; gains two
B) loses three; gains one
C) loses three; gains three
D) loses one; gains three
A) loses one; gains two
B) loses three; gains one
C) loses three; gains three
D) loses one; gains three

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
In which of the following pairings is the formula not consistent with the ions shown?
A) K2O (K+ and O-)
B) BaF2 (Ba2+ and F-)
C) Co2S3 (Co+3 and S-2)
D) Na3P (Na+ and P3-)
A) K2O (K+ and O-)
B) BaF2 (Ba2+ and F-)
C) Co2S3 (Co+3 and S-2)
D) Na3P (Na+ and P3-)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
The correct formula for the ionic compound containing Ni2+ and NO3? ions is ________.
A) NiNO3
B) Ni2NO3
C) Ni(NO3)3
D) Ni(NO3)2
A) NiNO3
B) Ni2NO3
C) Ni(NO3)3
D) Ni(NO3)2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
The correct formula for the ionic compound formed between Ca and I is ________.
A) Ca2I
B) CaI2
C) Ca2I3
D) Ca3I2
A) Ca2I
B) CaI2
C) Ca2I3
D) Ca3I2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
An ionic bond forms between two atoms through ________.
A) sharing of electron pairs
B) transferring of electrons from metallic atoms to nonmetallic atoms
C) transferring protons from the nucleus of the nonmetal to the nucleus of the metal
D) each atom acquiring a negative charge
A) sharing of electron pairs
B) transferring of electrons from metallic atoms to nonmetallic atoms
C) transferring protons from the nucleus of the nonmetal to the nucleus of the metal
D) each atom acquiring a negative charge

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following pairs of elements would most likely form a covalent compound?
A) Na and Pb
B) F and S
C) Cu and Ar
D) Zn and K
A) Na and Pb
B) F and S
C) Cu and Ar
D) Zn and K

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
Which statement is incorrect?
A) Ionic compounds generally contain metal and nonmetal elements.
B) Formulas of ionic compounds are written with the anion first, then the cation.
C) Cations and anions combine in the simplest ratio which achieves electrical neutrality.
D) The number of electrons lost by the cation(s) must equal the number gained by anion(s) in an ionic compound.
A) Ionic compounds generally contain metal and nonmetal elements.
B) Formulas of ionic compounds are written with the anion first, then the cation.
C) Cations and anions combine in the simplest ratio which achieves electrical neutrality.
D) The number of electrons lost by the cation(s) must equal the number gained by anion(s) in an ionic compound.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following formulas for an ionic compound is incorrect as written?
A) Al2(CO3)3
B) Na2S
C) Li2SO4
D) MgHCO3
A) Al2(CO3)3
B) Na2S
C) Li2SO4
D) MgHCO3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following formulas for an ionic compound is incorrect as written?
A) Ca(SO4)2
B) KOH
C) MgS
D) NH4ClO4
A) Ca(SO4)2
B) KOH
C) MgS
D) NH4ClO4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements is an accurate description of the structure of the ionic compound NaCl?
A) Alternating layers of Na and Cl molecules are present.
B) Alternating layers of Na+ and Cl- ions are present.
C) Alternating rows of Na+ and Cl- ions are present.
D) Each ion present is surrounded by six ions of opposite charge.
A) Alternating layers of Na and Cl molecules are present.
B) Alternating layers of Na+ and Cl- ions are present.
C) Alternating rows of Na+ and Cl- ions are present.
D) Each ion present is surrounded by six ions of opposite charge.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
A polyatomic ion is an ion that ________.
A) has a negative charge < -1
B) contains both a metal and a nonmetal
C) develops a charge as a result of the combination of two or more types of atoms
D) occurs alone as do molecules
A) has a negative charge < -1
B) contains both a metal and a nonmetal
C) develops a charge as a result of the combination of two or more types of atoms
D) occurs alone as do molecules

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
What is the correct chemical formula for a compound that contains K+ and CO32- ions?
A) K2CO3
B) KCO3
C) K(CO3)2
D) K3(CO3)2
A) K2CO3
B) KCO3
C) K(CO3)2
D) K3(CO3)2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
What is the formula for the ionic compound which forms when Co3+ combines with SO42-?
A) CoSO4
B) Co2(SO4)3
C) Co(SO4)2
D) Co3(SO4)2
A) CoSO4
B) Co2(SO4)3
C) Co(SO4)2
D) Co3(SO4)2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
For which of the following pairs of elements would "electron transfer" between atoms most likely occur?
A) Na and Cd
B) Ca and O
C) As and I
D) V and Li
A) Na and Cd
B) Ca and O
C) As and I
D) V and Li

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following pairs of elements would most likely form a polar covalent bond?
A) Se and Cl
B) Si and C
C) K and He
D) Ba and Ag
A) Se and Cl
B) Si and C
C) K and He
D) Ba and Ag

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the correct Lewis structure for OF2?
A)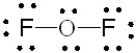
B)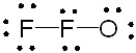
C)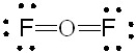
D)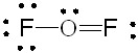
A)

B)
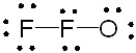
C)

D)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
The total number of "shared electron pairs" in the molecule H2S is ________.
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
Indicate the total number of electrons which would be shown as "dots" in a correctly written Lewis structure for OF2.
A) 18
B) 32
C) 26
D) 20
A) 18
B) 32
C) 26
D) 20

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following molecules contains a triple covalent bond?
A) Br2
B) C2H2Cl2
C) SO2
D) HCN
A) Br2
B) C2H2Cl2
C) SO2
D) HCN

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following general statements concerning covalent bond characteristics is incorrect?
A) Triple bonds are stronger than double bonds.
B) Double bonds are stronger than single bonds.
C) Double bonding can occur with Group VII A elements.
D) Triple bonding is possible when 3 or more electrons are needed to complete an octet.
A) Triple bonds are stronger than double bonds.
B) Double bonds are stronger than single bonds.
C) Double bonding can occur with Group VII A elements.
D) Triple bonding is possible when 3 or more electrons are needed to complete an octet.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
When drawing a Lewis structure, pairs of electrons that are not between atoms but are used to fill the octet of an atom are called ________.
A) bonding pairs
B) lone pairs
C) filled shells
D) excess electrons
A) bonding pairs
B) lone pairs
C) filled shells
D) excess electrons

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
Which bonding is not possible for carbon with its 4 valence electrons?
A) 4 single bonds
B) 2 single bonds and 1 double bond
C) 1 single bond and 1 triple bond
D) 1 double bond and 1 triple bond
A) 4 single bonds
B) 2 single bonds and 1 double bond
C) 1 single bond and 1 triple bond
D) 1 double bond and 1 triple bond

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
One of the resonance structures for the polyatomic ion NO3- is:
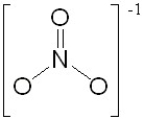
How many other resonance structures are there for this ion?
A) 1
B) 2
C) 3
D) 4

How many other resonance structures are there for this ion?
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
The following represents one of the resonance structures for the nitrite ion, NO2-.
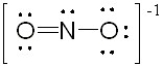
How many additional resonance structures can be drawn for NO2-?
A) 0
B) 1
C) 2
D) 3
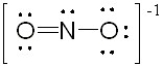
How many additional resonance structures can be drawn for NO2-?
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
How many resonance forms can be drawn for the CO3-2 ion?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
The Lewis structure for the SO42- ion contains how many "bonding electrons"?
A) 2
B) 4
C) 8
D) 12
A) 2
B) 4
C) 8
D) 12

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
In the Lewis structure of HCN, how many nonbonded electron pairs are present?
A) 3
B) 4
C) 2
D) 1
A) 3
B) 4
C) 2
D) 1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
In the Lewis structure, X represents a period 3 nonmetal. Identify the nonmetal.
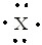
A) aluminum
B) sulfur
C) chlorine
D) nitrogen
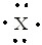
A) aluminum
B) sulfur
C) chlorine
D) nitrogen

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
Calculate the total number of electrons in the Lewis structure for the PH4+ ion.
A) 9
B) 8
C) 10
D) 12
A) 9
B) 8
C) 10
D) 12

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
Calculate the total number of electrons in the Lewis structure for the C2H3O2- ion.
A) 25
B) 32
C) 28
D) 24
A) 25
B) 32
C) 28
D) 24

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
The geometry associated with three pairs of bonded electrons and one pair of nonbonded electrons about a central atom in a molecule is ________.
A) tetrahedral
B) trigonal pyramidal
C) trigonal planar
D) angular
A) tetrahedral
B) trigonal pyramidal
C) trigonal planar
D) angular

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
The geometry of a molecule in which the central atom has 2 bonding electron pairs and 2 nonbonded electron pairs is ________.
A) linear
B) angular
C) trigonal planar
D) trigonal pyramidal
A) linear
B) angular
C) trigonal planar
D) trigonal pyramidal

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
The molecular shape (geometry) of a Cl2CO molecule is ________.
A) linear
B) trigonal pyramidal
C) trigonal planar
D) tetrahedral
A) linear
B) trigonal pyramidal
C) trigonal planar
D) tetrahedral

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
The molecular shape (geometry) of a NCl3 molecule is ________.
A) angular
B) trigonal planar
C) tetrahedral
D) trigonal pyramidal
A) angular
B) trigonal planar
C) tetrahedral
D) trigonal pyramidal

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
Which one of the following molecules is tetrahedral in shape?
A) XeF4
B) CF4
C) BF3
D) NH3
A) XeF4
B) CF4
C) BF3
D) NH3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
What is the molecular geometry of CO2?
A) linear
B) trigonal planar
C) tetrahedral
D) angular
A) linear
B) trigonal planar
C) tetrahedral
D) angular

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
The ability of an atom to attract electrons to itself in a molecule is called ________.
A) electron affinity
B) electronegativity
C) ionization energy
D) paramagnetism
A) electron affinity
B) electronegativity
C) ionization energy
D) paramagnetism

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following sets of elements is arranged in order of increasing electronegativity?
A) Cl, S, Se, Te
B) F, B, O, Li
C) Br, Cl, S, P
D) Fr, Mg, Si, F
A) Cl, S, Se, Te
B) F, B, O, Li
C) Br, Cl, S, P
D) Fr, Mg, Si, F

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
Which statement about electronegativity is incorrect?
A) Fluorine is the most electronegative atom of all the elements.
B) Metals generally have higher electronegativity values than nonmetals.
C) Within a periodic table row, electronegativity increases from left to right.
D) Within a periodic table group, electronegativity increases from bottom to top.
A) Fluorine is the most electronegative atom of all the elements.
B) Metals generally have higher electronegativity values than nonmetals.
C) Within a periodic table row, electronegativity increases from left to right.
D) Within a periodic table group, electronegativity increases from bottom to top.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
Which atom is the most electronegative?
A) Li
B) Cs
C) P
D) As
A) Li
B) Cs
C) P
D) As

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Which atom is the least electronegative?
A) F
B) Si
C) Rb
D) Ca
A) F
B) Si
C) Rb
D) Ca

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
If the electronegativity difference between two elements X and Y is 0.2, the bond between the two elements would be ________.
A) ionic
B) nonpolar covalent
C) polar covalent
D) coordinate covalent
A) ionic
B) nonpolar covalent
C) polar covalent
D) coordinate covalent

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following diatomic molecules contains the bond of greatest polarity?
A) P4
B) Cl-F
C) BrI
D) Te-F
A) P4
B) Cl-F
C) BrI
D) Te-F

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is the best notation for the bonding in the formula unit NaF?
A)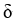 + 11eec96d_5bf8_46d2_b236_e5ca71510896_TB10281_11-
+ 11eec96d_5bf8_46d2_b236_e5ca71510896_TB10281_11-
Na-F
B) 11eec96d_5bf8_46d2_b236_e5ca71510896_TB10281_11- 11eec96d_5bf8_46d2_b236_e5ca71510896_TB10281_11+
Na-F
C) Na-F
D) Na+ F-
A)
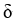 + 11eec96d_5bf8_46d2_b236_e5ca71510896_TB10281_11-
+ 11eec96d_5bf8_46d2_b236_e5ca71510896_TB10281_11-Na-F
B) 11eec96d_5bf8_46d2_b236_e5ca71510896_TB10281_11- 11eec96d_5bf8_46d2_b236_e5ca71510896_TB10281_11+
Na-F
C) Na-F
D) Na+ F-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
Which bond has the greatest percent covalent character?
A) O - F
B) Be - N
C) Cu - N
D) Li - Cl
A) O - F
B) Be - N
C) Cu - N
D) Li - Cl

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
For which of the bonds is the direction of polarity incorrect?
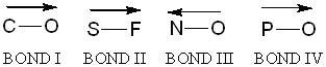
A) Bond I
B) Bond II
C) Bond III
D) Bond IV
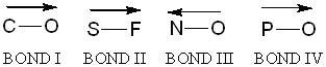
A) Bond I
B) Bond II
C) Bond III
D) Bond IV

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following bonds is the most polar?

A) As-Cl
B) N-F
C) I-Br
D) O-Si

A) As-Cl
B) N-F
C) I-Br
D) O-Si

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is a nonpolar molecule containing polar bonds?
A) H - H
B) CH4
C) F - Be - F
D) It is not possible for a molecule to be nonpolar while containing polar bonds.
A) H - H
B) CH4
C) F - Be - F
D) It is not possible for a molecule to be nonpolar while containing polar bonds.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements concerning molecular polarity is incorrect?
A) All A-B molecules are polar.
B) All linear X-A-X molecules are nonpolar.
C) All angular A-B-C molecules are nonpolar.
D) All symmetrical AX4 molecules are nonpolar.
A) All A-B molecules are polar.
B) All linear X-A-X molecules are nonpolar.
C) All angular A-B-C molecules are nonpolar.
D) All symmetrical AX4 molecules are nonpolar.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
Which molecule is not a polar molecule?
A) HCN
B) CHCl3
C) NH3
D) BCl3
A) HCN
B) CHCl3
C) NH3
D) BCl3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following molecules is polar?
A) BeCl2
B) BF3
C) CBr4
D) NCl3
A) BeCl2
B) BF3
C) CBr4
D) NCl3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
How many valence electrons do atoms with the following electron configurations have?
-1s22s22p63s23p64s23d104p4
-1s22s22p63s23p64s23d104p4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
How many valence electrons do atoms with the following electron configurations have?
-1s22s22p63s2
-1s22s22p63s2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
How many valence electrons do atoms with the following electron configurations have?
-1s22s22p63s23p64s1
-1s22s22p63s23p64s1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
How many valence electrons do atoms with the following electron configurations have?
-1s22s22p5
-1s22s22p5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
Determine the formula for the compound formed between the following pairs of elements and/or ions.
-Cs and P4
-Cs and P4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
78
Determine the formula for the compound formed between the following pairs of elements and/or ions.
-As and Cl2
-As and Cl2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
79
Determine the formula for the compound formed between the following pairs of elements and/or ions.
-OH− and Fe3+
-OH− and Fe3+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
80
Determine the formula for the compound formed between the following pairs of elements and/or ions.
-O2 and F2
-O2 and F2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck



