Deck 6: Electronic Structure and Chemical Periodicity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 6: Electronic Structure and Chemical Periodicity
1
The periodic law states that when elements are arranged in order of ________ their properties repeat themselves at regular intervals.
A) increasing atomic number
B) decreasing atomic number
C) increasing atomic mass
D) decreasing atomic mass
A) increasing atomic number
B) decreasing atomic number
C) increasing atomic mass
D) decreasing atomic mass
increasing atomic number
2
Elements constituting a period in the periodic table ________.
A) have similar chemical properties
B) are called isotopes
C) have consecutive atomic numbers
D) will always be in the same group
A) have similar chemical properties
B) are called isotopes
C) have consecutive atomic numbers
D) will always be in the same group
have consecutive atomic numbers
3
In which of the following sets of elements are all members of the set in the same group in the periodic table?
A) 9F, 10Ne, and 11Na
B) 31Ga, 49In, and 81Tl
C) 14Si, 15P, and 16S
D) 20Ca, 26Fe, and 34Se
A) 9F, 10Ne, and 11Na
B) 31Ga, 49In, and 81Tl
C) 14Si, 15P, and 16S
D) 20Ca, 26Fe, and 34Se
31Ga, 49In, and 81Tl
4
Which one of the following elements occupies position period 5 and group IIA in the periodic table?
A) B
B) Y
C) Sr
D) Kr
A) B
B) Y
C) Sr
D) Kr

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following elements occupies position Period 5 and Group IIIA in the periodic table?
A) P
B) In
C) As
D) Tl
A) P
B) In
C) As
D) Tl

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
Group IA elements are called:
A) alkali metals
B) alkaline earth metals
C) noble gases
D) halogens
A) alkali metals
B) alkaline earth metals
C) noble gases
D) halogens

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
The elements in groups IA, VIIA and VIIIA are called, respectively:
A) alkaline earth metals, halogens, chalcogens
B) alkali metals, chalcogens, halogens
C) alkali metals, halogens, noble gases
D) alkaline earth metals, transition metals, halogens
A) alkaline earth metals, halogens, chalcogens
B) alkali metals, chalcogens, halogens
C) alkali metals, halogens, noble gases
D) alkaline earth metals, transition metals, halogens

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a quantized property of an electron?
A) energy
B) nuclear charge
C) number
D) charge to mass ratio
A) energy
B) nuclear charge
C) number
D) charge to mass ratio

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
The maximum number of electrons found in a shell ________.
A) is the same as the shell number
B) doubles as the shell number increases by one
C) varies in an unpredictable manner
D) is equal to 2n2
A) is the same as the shell number
B) doubles as the shell number increases by one
C) varies in an unpredictable manner
D) is equal to 2n2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
What is the maximum number of electrons in the n = 4 shell?
A) 16
B) 8
C) 18
D) 32
A) 16
B) 8
C) 18
D) 32

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
Which response includes all the following statements that are true, and no others?
I. The f subshell contains 7 orbitals.
II. The third energy shell (n=3) has no f orbitals.
III. There are ten d orbitals in the d subshell.
IV. The second energy shell contains only s and p orbitals.
V. An orbital can accommodate a maximum of 2 electrons.
A) I, II, and IV
B) II, III, and V
C) II and IV
D) I, II, IV, and V
I. The f subshell contains 7 orbitals.
II. The third energy shell (n=3) has no f orbitals.
III. There are ten d orbitals in the d subshell.
IV. The second energy shell contains only s and p orbitals.
V. An orbital can accommodate a maximum of 2 electrons.
A) I, II, and IV
B) II, III, and V
C) II and IV
D) I, II, IV, and V

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following subshells is lowest in energy?
A) 6s
B) 3d
C) 4s
D) 5d
A) 6s
B) 3d
C) 4s
D) 5d

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
How many subshells are in the n = 3 shell?
A) 2
B) 3
C) 4
D) 5
A) 2
B) 3
C) 4
D) 5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
How many subshells are present in the third electron shell?
A) 1
B) 2
C) 4
D) 3
A) 1
B) 2
C) 4
D) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
In the ground state of an atom, ________.
A) the excited states are all filled
B) all the electrons are in their lowest energy levels
C) the protons and neutrons fill all the available energy states
D) the energy of the electrons are at a maximum
A) the excited states are all filled
B) all the electrons are in their lowest energy levels
C) the protons and neutrons fill all the available energy states
D) the energy of the electrons are at a maximum

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
The term "main energy level" is closely associated with the term ________.
A) supershell
B) shell
C) subshell
D) orbital
A) supershell
B) shell
C) subshell
D) orbital

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements concerning electron subshells is correct?
A) Electrons in a subshell have equal energies.
B) The number of subshells in a shell is equal to S2.
C) Subshells are identified by a whole number integer only.
D) A "p" subshell exists in all shells.
A) Electrons in a subshell have equal energies.
B) The number of subshells in a shell is equal to S2.
C) Subshells are identified by a whole number integer only.
D) A "p" subshell exists in all shells.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements about a d subshell is incorrect?
A) It contains 5 orbitals.
B) It may contain a maximum of 10 electrons.
C) Is found in the 3rd energy level only.
D) It is found in all energy levels where n=3.
A) It contains 5 orbitals.
B) It may contain a maximum of 10 electrons.
C) Is found in the 3rd energy level only.
D) It is found in all energy levels where n=3.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
The shape of an orbital is most closely related to which of the following factors?
A) the average energy of electrons within the shell containing the orbital
B) the shell in which the orbital is located
C) the type of subshell in which the orbital is located
D) the number of electrons within the orbital
A) the average energy of electrons within the shell containing the orbital
B) the shell in which the orbital is located
C) the type of subshell in which the orbital is located
D) the number of electrons within the orbital

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
Which statement about orbitals is incorrect?
A) Different subshells have different shapes.
B) Two electrons can occupy each lobe of a p orbital.
C) At a given time, an electron can only be at one point in an orbital.
D) An orbital is a region of space where an electron is most likely to be found.
A) Different subshells have different shapes.
B) Two electrons can occupy each lobe of a p orbital.
C) At a given time, an electron can only be at one point in an orbital.
D) An orbital is a region of space where an electron is most likely to be found.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
What is the maximum number of electrons that the "d" subshell can hold?
A) 32
B) 10
C) 5
D) 6
A) 32
B) 10
C) 5
D) 6

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
What is the maximum number of orbitals possible for the third principal energy level of an atom?
A) 5
B) 8
C) 10
D) 9
A) 5
B) 8
C) 10
D) 9

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
The atomic orbital depicted is a(n) ________ orbital.

A) d
B) s
C) p
D) f

A) d
B) s
C) p
D) f

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
The atomic orbital depicted below would be found in a ________ subshell.
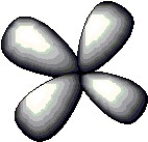
A) 3p
B) 2s
C) 5d
D) 4f

A) 3p
B) 2s
C) 5d
D) 4f

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a representation for an p orbital?
A)
B)
C)
D)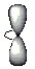
A)

B)

C)

D)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following subshell notations for electron occupancy is an impossibility?
A) 5s3
B) 4p5
C) 4f11
D) 2p1
A) 5s3
B) 4p5
C) 4f11
D) 2p1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
In an atom with many electrons, which of the following orbitals would be highest in energy?
A) 7s
B) 6d
C) 4p
D) 4f
A) 7s
B) 6d
C) 4p
D) 4f

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
After the 5s subshell of an atom is filled with electrons, the next electron added will enter the ________.
A) 5p subshell
B) 4d subshell
C) 4p subshell
D) 5f subshell
A) 5p subshell
B) 4d subshell
C) 4p subshell
D) 5f subshell

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
Indicate the missing words in the following statement: The Aufbau principle states that ________ normally occupy the lowest energy subshell available.
A) nucleus
B) electrons
C) protons
D) neutrons
A) nucleus
B) electrons
C) protons
D) neutrons

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
The correct electron configuration for chromium is ________.
A) 1s22s22p63s23p64s13d5
B) 1s22s22p63s23p63d6
C) 1s22s22p63s23p6
D) 1s22s22p63s23p64s23d104p1
A) 1s22s22p63s23p64s13d5
B) 1s22s22p63s23p63d6
C) 1s22s22p63s23p6
D) 1s22s22p63s23p64s23d104p1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
The electron configuration 1s22s22p63s23p64s23d104p65s24d105p2 is for the element ________.
A) Sb
B) Pb
C) As
D) Sn
A) Sb
B) Pb
C) As
D) Sn

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
How many electrons are there in the outermost shell and subshell, respectively, in an atom with the electron configuration 1s22s22p63s23p64s23d104p1?
A) 1, 10
B) 2, 2
C) 5, 3
D) 3, 1
A) 1, 10
B) 2, 2
C) 5, 3
D) 3, 1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
The element with the electron configuration given below is ________.
1s22s22p63s23p64s23d104p65s24d1
A) Sc
B) Si
C) La
D) Y
1s22s22p63s23p64s23d104p65s24d1
A) Sc
B) Si
C) La
D) Y

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following electron configurations is incorrect as written?
A) 1s22s22p63s23p64s23d104p4
B) 1s22s22px22py22px23s2
C) 1s22s22p63s23p63d10
D) 1s22s22p63s2
A) 1s22s22p63s23p64s23d104p4
B) 1s22s22px22py22px23s2
C) 1s22s22p63s23p63d10
D) 1s22s22p63s2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
Hund's rule is needed in drawing an orbital diagram for which of the following elements?
A) krypton
B) lithium
C) boron
D) selenium
A) krypton
B) lithium
C) boron
D) selenium

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
The number of unpaired electrons present in a magnesium atom is ________.
A) 0
B) 1
C) 3
D) 5
A) 0
B) 1
C) 3
D) 5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
How many unpaired electrons are in a Ru atom?
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
In which pair are the atoms, Ba and Xe, correctly identified regarding their magnetic properties?
A) Ba: diamagnetic
Xe: diamagnetic
B) Ba: diamagnetic
Xe: paramagnetic
C) Ba: paramagnetic
Xe: diamagnetic
D) Ba: paramagnetic
Xe: paramagnetic
A) Ba: diamagnetic
Xe: diamagnetic
B) Ba: diamagnetic
Xe: paramagnetic
C) Ba: paramagnetic
Xe: diamagnetic
D) Ba: paramagnetic
Xe: paramagnetic

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
In the electron configuration of an arsenic atom, there are ________ unpaired electrons.
A) 2
B) 0
C) 1
D) 3
A) 2
B) 0
C) 1
D) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
How many unpaired electrons are there in a phosphorous atom?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
How many unpaired electrons are there in a oxygen atom?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
Which orbital diagram is incorrect according to Hund's Rule? 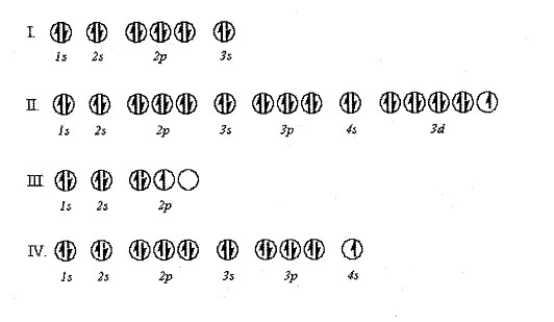
A) Diagram I
B) Diagram II
C) Diagram III
D) Diagram IV

A) Diagram I
B) Diagram II
C) Diagram III
D) Diagram IV

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
How many unpaired electrons would be present in an orbital diagram for a strontium atom?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
Which element is represented by the electronic orbital diagram given? 
A) phosphorus
B) sulfur
C) chlorine
D) selenium

A) phosphorus
B) sulfur
C) chlorine
D) selenium

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following elements would be in the same group of the periodic table as the element whose configuration is
1s22s22p63s23p64s1?
A) 3Li
B) 15P
C) 18Ar
D) 34Se
1s22s22p63s23p64s1?
A) 3Li
B) 15P
C) 18Ar
D) 34Se

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
All of the elements in Group VIIA have an electron configuration that ends in ________.
A) p6
B) s2
C) d10
D) p5
A) p6
B) s2
C) d10
D) p5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following pairings is incorrect?
A) Au - d area of periodic table
B) Ir - f area of periodic table
C) Xe - p area of periodic table
D) Be - s area of periodic table
A) Au - d area of periodic table
B) Ir - f area of periodic table
C) Xe - p area of periodic table
D) Be - s area of periodic table

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following elements has an electron configuration ending in 4f3?
A) 40Zr
B) 60Nd
C) 73Ta
D) 72Hf
A) 40Zr
B) 60Nd
C) 73Ta
D) 72Hf

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
The distinguishing electron is in the same type of subshell for which of the following pairs of elements?
A) 3Li and 48Cd
B) 17Cl and 30Zn
C) 51Sb and 16S
D) 77Ir and 20Ca
A) 3Li and 48Cd
B) 17Cl and 30Zn
C) 51Sb and 16S
D) 77Ir and 20Ca

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
An atom of an element has an electron configuration ending in 3p4. Which of the following statements about the element's electron configuration is incorrect?
A) There are eight electrons in the 2nd shell.
B) The 3rd shell needs two more electrons to be completely filled.
C) There are six electrons in the 3rd shell.
D) Five different subshells contain electrons.
A) There are eight electrons in the 2nd shell.
B) The 3rd shell needs two more electrons to be completely filled.
C) There are six electrons in the 3rd shell.
D) Five different subshells contain electrons.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following elements is an alkaline earth metal?
A) 38Sr
B) 21Sc
C) 34Se
D) 54Xe
A) 38Sr
B) 21Sc
C) 34Se
D) 54Xe

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following element-classification pairings is incorrect?
A) K - representative element
B) Ar - noble gas
C) Mo - transition element
D) Po - halogen
A) K - representative element
B) Ar - noble gas
C) Mo - transition element
D) Po - halogen

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
In which of the following pairs of elements is one element a metalloid and one element a nonmetal?
A) 33As and 14Si
B) 51Sb and 20Ca
C) 32Ge and 9F
D) 82Pb and 83Bi
A) 33As and 14Si
B) 51Sb and 20Ca
C) 32Ge and 9F
D) 82Pb and 83Bi

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
The number of known nonmetals is ________ the number of known metals.
A) about 1/4 that of
B) very small compared to
C) about four times greater than
D) about double
A) about 1/4 that of
B) very small compared to
C) about four times greater than
D) about double

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
In which of the following areas of the periodic table do you find both metals and nonmetals (ignoring the position of hydrogen)?
A) both s and p areas
B) s area only
C) p area only
D) both p and d areas
A) both s and p areas
B) s area only
C) p area only
D) both p and d areas

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
Which column of the periodic table has 3 nonmetal and 2 metalloid elements?
A) Group III A
B) Group IV A
C) Group V A
D) Group VI A
A) Group III A
B) Group IV A
C) Group V A
D) Group VI A

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
Which type of subshell is filled by the distinguishing electron of an alkaline earth metal?
A) d
B) s
C) p
D) f
A) d
B) s
C) p
D) f

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following statements concerning periodic trends is correct?
A) Metallic character decreases in moving from right to left across a given period in the periodic table.
B) Nonmetallic character increases in moving from top to bottom down a group in the periodic table.
C) Atomic radii tend to decrease from left to right in a period of the periodic table.
D) Atomic radii tend to decrease from top to bottom within a periodic table group.
A) Metallic character decreases in moving from right to left across a given period in the periodic table.
B) Nonmetallic character increases in moving from top to bottom down a group in the periodic table.
C) Atomic radii tend to decrease from left to right in a period of the periodic table.
D) Atomic radii tend to decrease from top to bottom within a periodic table group.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
The largest atoms are found where in the periodic table?
A) in the transition metals
B) in the lower left
C) in the upper left
D) in the lower right
A) in the transition metals
B) in the lower left
C) in the upper left
D) in the lower right

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is not a metallic property?
A) shiny
B) conducting
C) malleable
D) brittle
A) shiny
B) conducting
C) malleable
D) brittle

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following elements would be expected to have the greatest nonmetallic character?
A) Al
B) Ga
C) In
D) B
A) Al
B) Ga
C) In
D) B

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following elements has the largest atomic radius?
A) 11Na
B) 13Al
C) 15P
D) 17Cl
A) 11Na
B) 13Al
C) 15P
D) 17Cl

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following elements is a metal?
A) 14Si
B) 13Al
C) 16S
D) 33As
A) 14Si
B) 13Al
C) 16S
D) 33As

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
Which is a characteristic property of nonmetals?
A) brittle
B) malleability
C) thermal conductivity
D) luster
A) brittle
B) malleability
C) thermal conductivity
D) luster

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following elements would exhibit chemical properties most similar to nitrogen?
A) Ge
B) Zn
C) Sb
D) Ne
A) Ge
B) Zn
C) Sb
D) Ne

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following elements is a metalloid
A) Al
B) Si
C) N
D) Mg
A) Al
B) Si
C) N
D) Mg

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
Which element is the most metallic?
A) fluorine
B) barium
C) zinc
D) scandium
A) fluorine
B) barium
C) zinc
D) scandium

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following atomic numbers is that of a noble gas?
A) 11
B) 32
C) 24
D) 10
A) 11
B) 32
C) 24
D) 10

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
Arrange the following elements in order of increasing atomic radius.
Sr, Rb, Sb, I, In
A) Rb < Sr < In < Sb < I
B) I < Sb < In < Rb < Sr
C) In < Sb < I < Sr < Rb
D) I < Sb < In < Sr < Rb
Sr, Rb, Sb, I, In
A) Rb < Sr < In < Sb < I
B) I < Sb < In < Rb < Sr
C) In < Sb < I < Sr < Rb
D) I < Sb < In < Sr < Rb

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
Rank the elements below in order of decreasing atomic radius.
Mg, Na, P, Si, Ar
A) Mg, Na, P, Si, Ar
B) Na, Mg, Si, P, Ar
C) Si, P, Ar, Na, Mg
D) Ar, Si, P, Na, Mg
Mg, Na, P, Si, Ar
A) Mg, Na, P, Si, Ar
B) Na, Mg, Si, P, Ar
C) Si, P, Ar, Na, Mg
D) Ar, Si, P, Na, Mg

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
Indicate whether the following statements are true or false.
-An electron that has n = 5 could be in a s , p, d, or f sublevel.
-An electron that has n = 5 could be in a s , p, d, or f sublevel.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
Indicate whether the following statements are true or false.
-The maximum number of electrons accommodated by the 4th energy shell (n = 4) is 32.
-The maximum number of electrons accommodated by the 4th energy shell (n = 4) is 32.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
Indicate whether the following statements are true or false.
-The third energy shell contains a total of 10 orbitals.
-The third energy shell contains a total of 10 orbitals.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
Circle the correct symbol or the formula which has:
-Electron configuration ends in 5p3
A) 33As
B) 51Sb
C) 73Ta
D) 83Bi
-Electron configuration ends in 5p3
A) 33As
B) 51Sb
C) 73Ta
D) 83Bi

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
Circle the correct symbol or the formula which has:
-1s22s22p63s23p64s23d104p6
A) 36Kr
B) 34Se
C) 38Sr
D) 52Te
-1s22s22p63s23p64s23d104p6
A) 36Kr
B) 34Se
C) 38Sr
D) 52Te

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
Circle the correct symbol or the formula which has:
-4f subshell begins filling
A) 37Rb
B) 55Cs
C) 58Ce
D) 87Fr
-4f subshell begins filling
A) 37Rb
B) 55Cs
C) 58Ce
D) 87Fr

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
Circle the correct symbol or the formula which has:
-2nd energy shell contains 4 electrons
A) 6C
B) 14Si
C) 4Be
D) 14Si.
-2nd energy shell contains 4 electrons
A) 6C
B) 14Si
C) 4Be
D) 14Si.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
78
Circle the correct symbol or the formula which has:
-3rd shell becomes completely filled
A) Mg
B) Hg
C) Zn
D) Cd
-3rd shell becomes completely filled
A) Mg
B) Hg
C) Zn
D) Cd

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
79
Circle the correct symbol or the formula which has:
-Number of electrons in the 3d subshell of Mn
A) 0
B) 5
C) 7
D) 10
-Number of electrons in the 3d subshell of Mn
A) 0
B) 5
C) 7
D) 10

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
80
Circle the correct symbol or the formula which has:
-Contains a total of twenty-one "p" electrons
A) 36Kr
B) 51Sb
C) 54Xe
D) 83Bi
-Contains a total of twenty-one "p" electrons
A) 36Kr
B) 51Sb
C) 54Xe
D) 83Bi

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck



