Deck 1: Structure Determines Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/15
Play
Full screen (f)
Deck 1: Structure Determines Properties
1
What is the ground state electron configuration of carbon?
A)
B)
C)
D)
A)
B)
C)
D)
2
Which one of the following is the conjugate base of ?
A)
B)
C)
D)
A)
B)
C)
D)
3
Identify the condensed formula of the following structure:
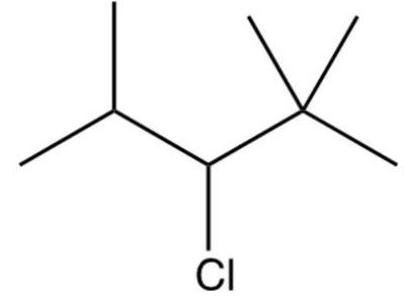
A)
B)
C)
D)

A)
B)
C)
D)
4
How many constitutional isomers are possible?
A) one
B) two
C) three
D) four
A) one
B) two
C) three
D) four

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
5
The formal charges on the nitrogen and oxygen in the following structures are, respectively
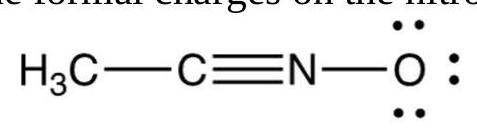
A)
B)
C)
D) 0,0
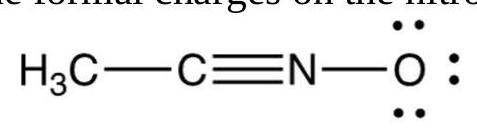
A)
B)
C)
D) 0,0

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
6
In which of the following compounds would you expect to have a partial positive charge?
A)
B)
C)
D)
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following species has(have) a linear geometry?
I.
II.
III.
A) only I
B) only II
C) I and II
D) I, II, and III
I.
II.
III.
A) only I
B) only II
C) I and II
D) I, II, and III

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
8
The bond angles in ethylene, , are closest to
A) .
B) .
C) .
D) .
A) .
B) .
C) .
D) .

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
9
Give the molecular formula of the compound shown below:
A)
B)
C)
D)

A)
B)
C)
D)

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following is the conjugate acid of ethanol?

A) Option A
B) Option B
C) Option C
D) Option D

A) Option A
B) Option B
C) Option C
D) Option D

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
11
In the equilibrium below, the strongest base is:
A)
B)
C)
D)
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
12
Which one of the following mechanistically depicts the protonation of methanol by hydrogen bromide?
A)
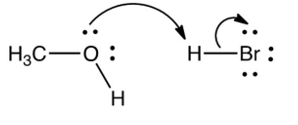
B)
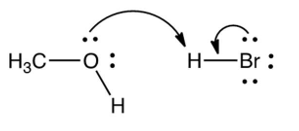
C)
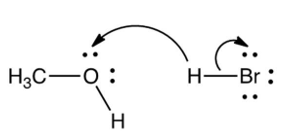
D)
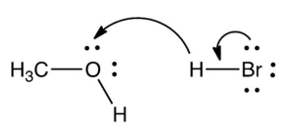
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following has the largest acid equilibrium constant, ?
A)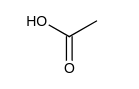
B)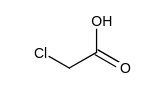
C)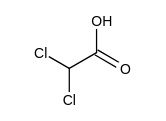
D)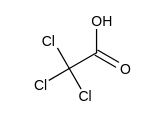
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
14
For which of the following does the equilibrium favor reactants.
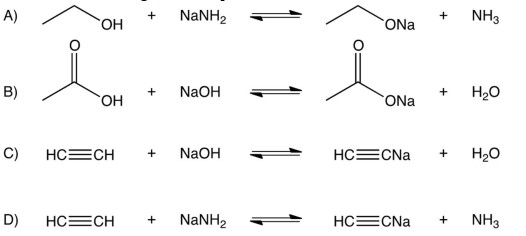
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the resonance structure which results from the following "electron pair movements".
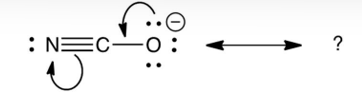
A)
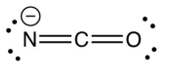
B)
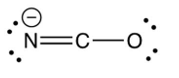
C)
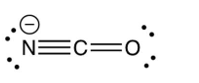
D)
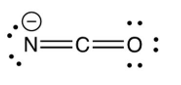

A)
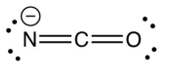
B)
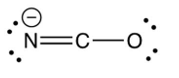
C)
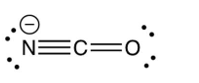
D)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck



