Deck 14: Organometallic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/13
Play
Full screen (f)
Deck 14: Organometallic Compounds
1
Which one of the following would not be a suitable solvent for Grignard reagents?
A)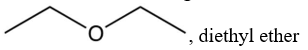
B)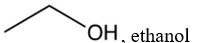
C)
D) they would all be suitable solvents.
A)

B)

C)

D) they would all be suitable solvents.

2
What is the major product of the following reaction?
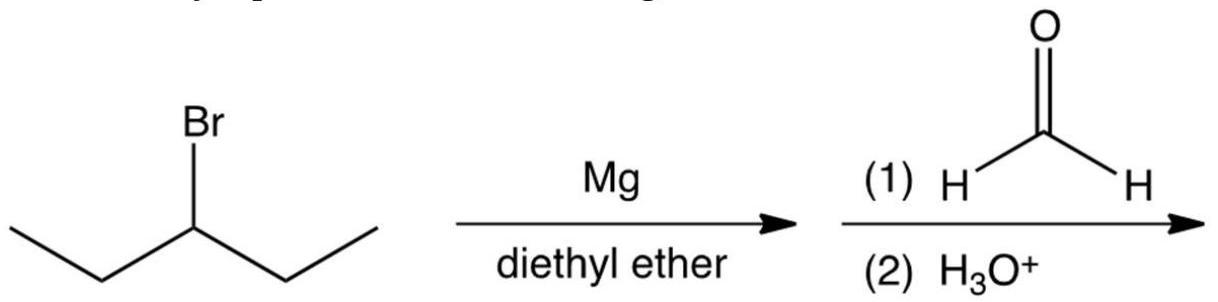
A) 2-ethyl-1-pentanol
B) 2-ethyl-1-butanol
C) 3-pentanol
D) 3-methyl-1-pentanol

A) 2-ethyl-1-pentanol
B) 2-ethyl-1-butanol
C) 3-pentanol
D) 3-methyl-1-pentanol
2-ethyl-1-butanol
3
The reaction of excess Grignard reagent with an ester of formic acid, , gives
A) a primary alcohol.
B) a secondary alcohol.
C) a tertiary alcohol.
D) methanol.
A) a primary alcohol.
B) a secondary alcohol.
C) a tertiary alcohol.
D) methanol.
a secondary alcohol.
4
Which of the following pairs of reagents would you use to prepare 4-methyl-2-pentanol?
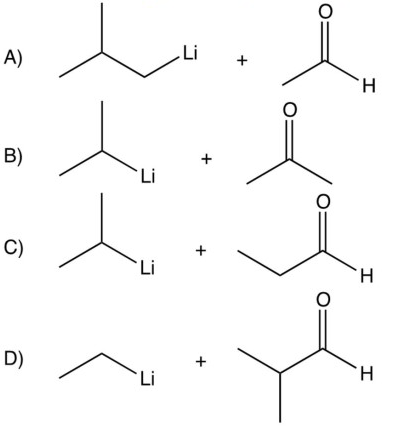
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
5
What is the product of the following reactions?
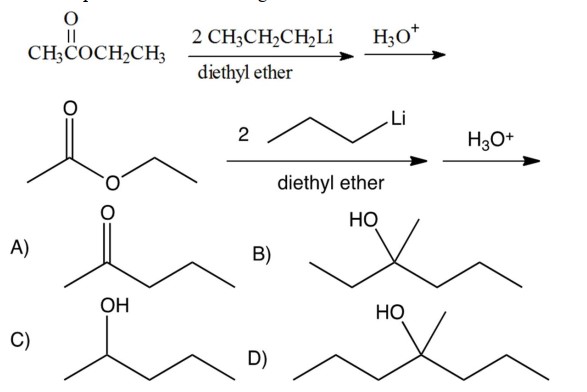
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
6
The reaction of 4-methylcyclohexanone with followed by neutralization gives two alcohols. These two alcohols are
A) constitutional isomers.
B) enantiomers formed in equal amounts.
C) enantiomers formed in unequal amounts.
D) diastereomers.
A) constitutional isomers.
B) enantiomers formed in equal amounts.
C) enantiomers formed in unequal amounts.
D) diastereomers.

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
7
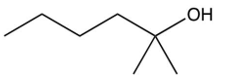
What is the product of the following reaction?
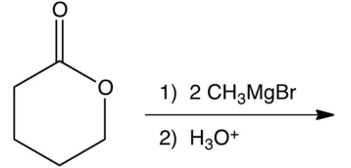
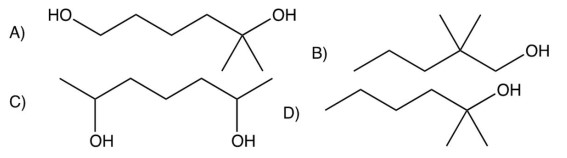
A)
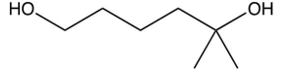
B)
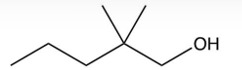
C)
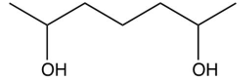
D)


A)

B)

C)

D)


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the two syntheses of the compound shown below. Which method would work best with minimal by-products?
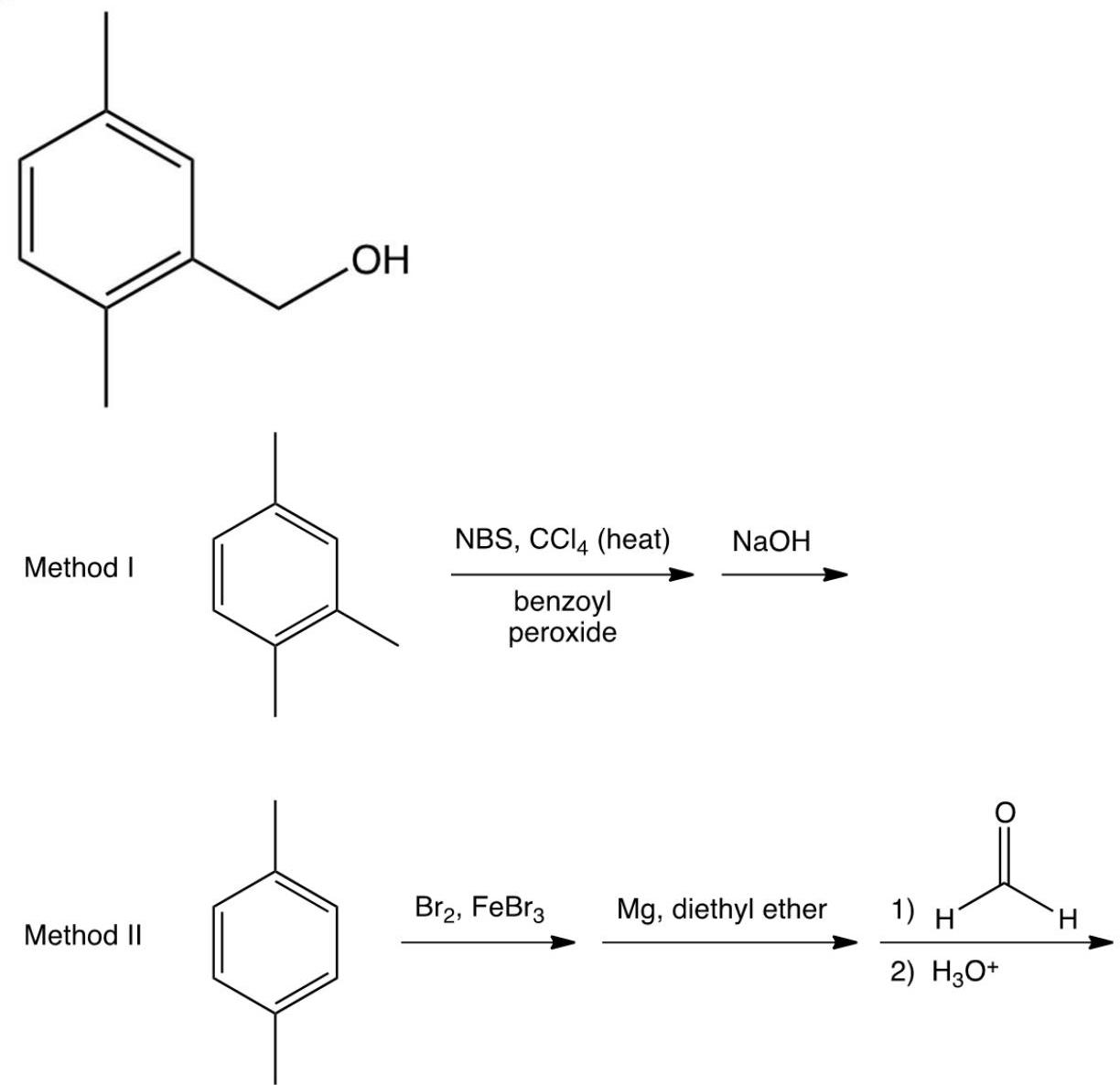
A) Method I
B) Method II
C) Both methods would work.
D) Neither method would work.

A) Method I
B) Method II
C) Both methods would work.
D) Neither method would work.

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
9
What is the product of the following reaction?
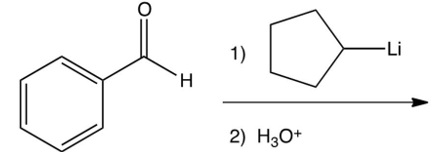
A)
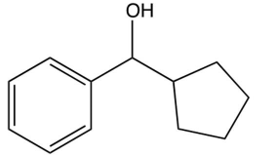
B)
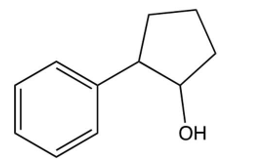
C)
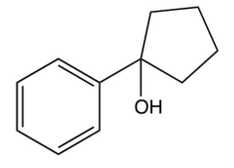
D)
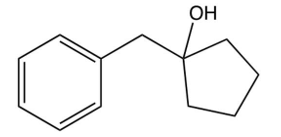

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
10
The reaction of a Grignard reagent with a ketone followed by dilute acid gives a(n)
A) primary alcohol.
B) secondary alcohol.
C) tertiary alcohol.
D) ester.
A) primary alcohol.
B) secondary alcohol.
C) tertiary alcohol.
D) ester.

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
11
What is the product of the following reaction sequence?
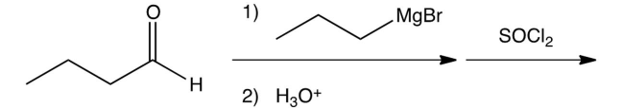
A) 3-chloro-4-heptanol
B) 3-heptene
C) 3-chloroheptane
D) 4-chloroheptane

A) 3-chloro-4-heptanol
B) 3-heptene
C) 3-chloroheptane
D) 4-chloroheptane

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
12
How many stereoisomers are formed in this reaction?
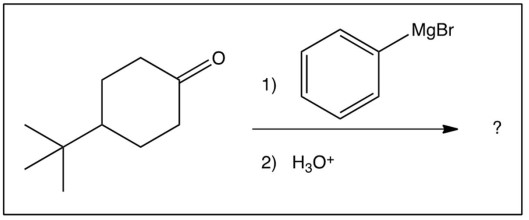
A) just one
B) two
C) three
D) four

A) just one
B) two
C) three
D) four

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
13
What organic product would result from this reaction?

A)
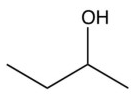
B)
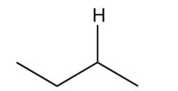
C)
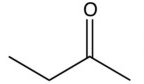
D)
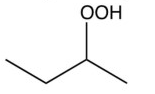

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck



