Deck 15: Alcohols, Diols, and Thiols
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/15
Play
Full screen (f)
Deck 15: Alcohols, Diols, and Thiols
1
What is the product of the following reactions?
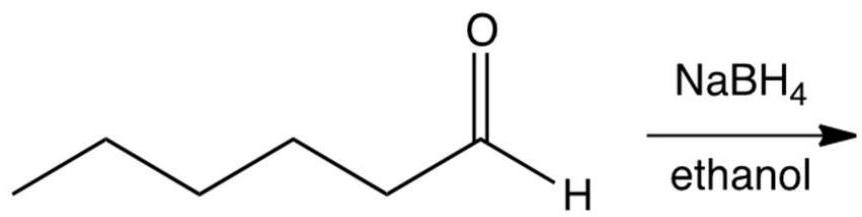
A)
B)
C)
D)

A)
B)
C)
D)
2
Which of the following cannot be made by the reduction of a ketone or aldehyde with in methanol?
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
2-methyl-2-propanol
3
What is the product of the following reaction?
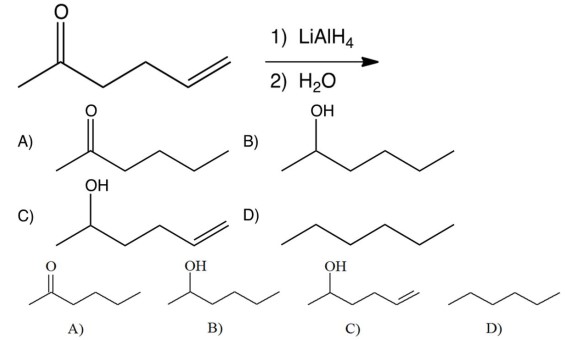
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D
C
4
Give the product of the following reaction.
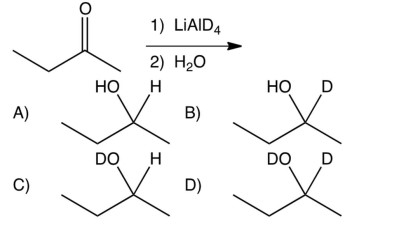
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
5
The reaction of a Grignard reagent with ethylene oxide followed by dilute acid gives
A) a primary alcohol.
B) a secondary alcohol.
C) a tertiary alcohol.
D) methanol.
A) a primary alcohol.
B) a secondary alcohol.
C) a tertiary alcohol.
D) methanol.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the conversion of 1-butanol to each of the compounds shown below. In which conversion is an oxidizing agent needed?
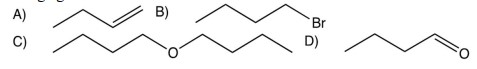
A)
B)
C)
D)

A)
B)
C)
D)

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following syntheses gives 3-methyl-1-hexanol?
A)
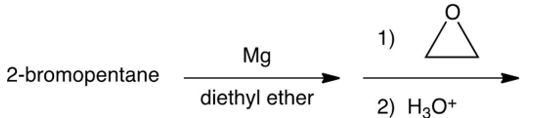
B)
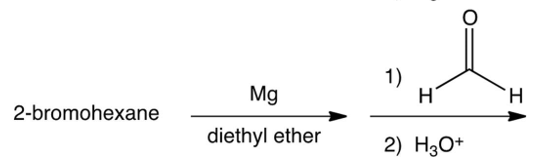
C)
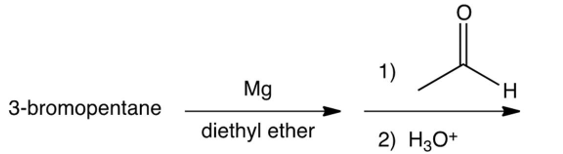
D)
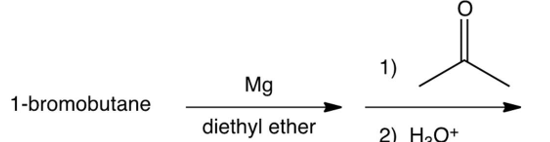
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
8
What is the product of the synthetic sequence below?
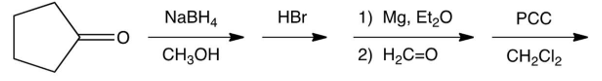
A)
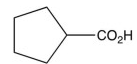
B)
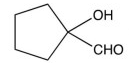
C)
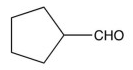
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
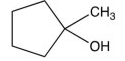
9
Compound , is readily oxidized with in to give compound . Compound has four peaks in its C-13 NMR (broadband decoupled). Which one of the following fits the data for compound ?
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
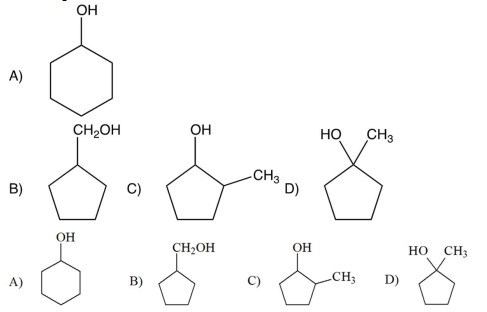
10
What is the final product of the following reactions?
A) 2,3-dimethyl-3-pentanol
B) 2,3-dimethyl-2-pentanol
C) 2,4-dimethyl-3-pentanol
D) 2,2-dimethyl-3-pentanol

A) 2,3-dimethyl-3-pentanol
B) 2,3-dimethyl-2-pentanol
C) 2,4-dimethyl-3-pentanol
D) 2,2-dimethyl-3-pentanol

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
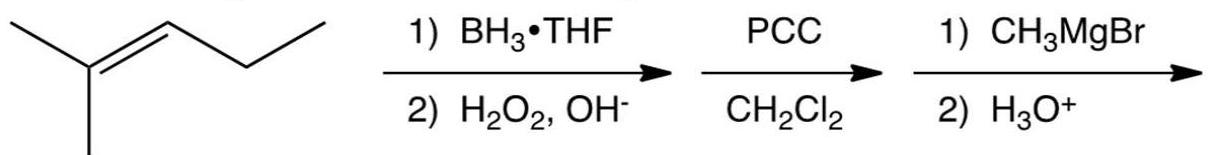
11
What is the product of the reaction below?
A)
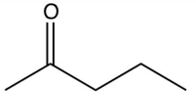
B)
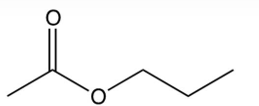
C)
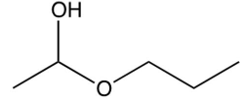
D)
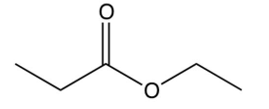

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
12
What is the product of the following reaction sequence?
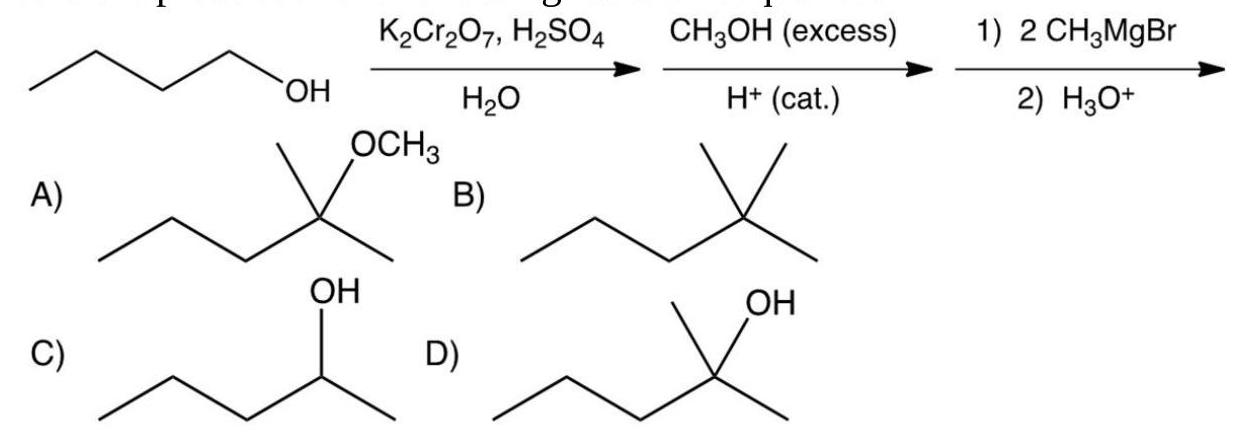
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
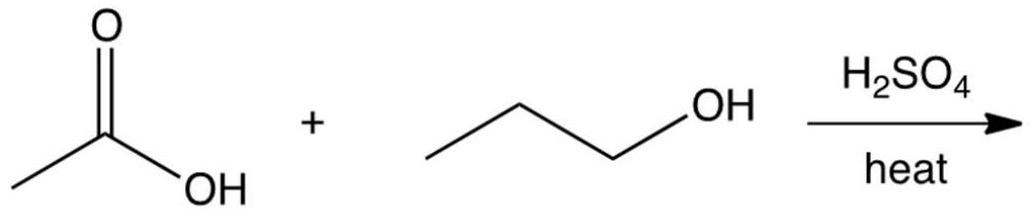
13
Which one of the following reaction steps work best to carry out the transformation shown below?
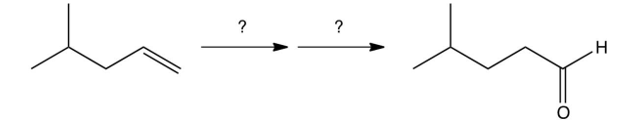
A)
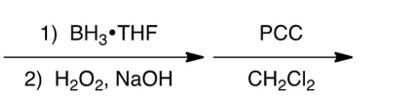
B)
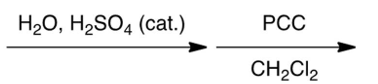
C)
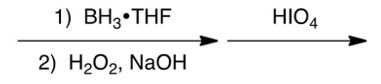
D)
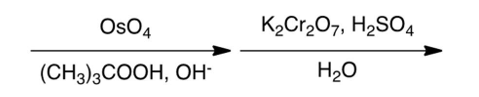

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
14
What is the product of the following reaction sequence?
Cyclopentanone
A) cyclopentene oxide
B) cyclopentene
C) cyclopentane
D) cis-1,2-cyclopentanediol
Cyclopentanone
A) cyclopentene oxide
B) cyclopentene
C) cyclopentane
D) cis-1,2-cyclopentanediol

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
15
To which side, if any, would the reaction below lie?
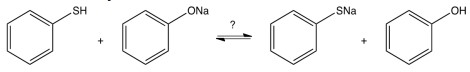
A) to the right
B) to the left
C) equally to both sides
D) this reaction cannot occur

A) to the right
B) to the left
C) equally to both sides
D) this reaction cannot occur

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck



