Deck 11: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/12
Play
Full screen (f)
Deck 11: Electrochemistry
1
An electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
-Refer to Exhibit 17-2. What is the amount of electrical work done by the battery?
A) -72900 J
B) 72900 J
C) 122 J
D) -122 J
E) Not enough information provided
F) None of the above
-Refer to Exhibit 17-2. What is the amount of electrical work done by the battery?
A) -72900 J
B) 72900 J
C) 122 J
D) -122 J
E) Not enough information provided
F) None of the above
72900 J
2
An electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
-Refer to Exhibit 17-2. How many moles of electrons flow through the wire?
A) 96485
B) 0.756
C) 0.504
D) 1.40´10-4
E) None of the above
-Refer to Exhibit 17-2. How many moles of electrons flow through the wire?
A) 96485
B) 0.756
C) 0.504
D) 1.40´10-4
E) None of the above
0.504
3
An electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
-Refer to Exhibit 17-2. If the iron electrode initially weighed 20.00 g, what is the mass after the flow of current is stopped?
A) 34.79 g
B) 34.07 g
C) 49.57 g
D) 5.22 g
E) 5.93 g
-Refer to Exhibit 17-2. If the iron electrode initially weighed 20.00 g, what is the mass after the flow of current is stopped?
A) 34.79 g
B) 34.07 g
C) 49.57 g
D) 5.22 g
E) 5.93 g
34.79 g
4
The electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)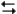 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
-Refer to Exhibit 17-3. What is the value of "n" used in the Nernst equation for the reaction above?
A) 1
B) 2
C) 3
D) Not enough data given to answer the question
E) None of the above
Ag+(0.275 M) + Fe2+(0.180 M)
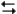 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)-Refer to Exhibit 17-3. What is the value of "n" used in the Nernst equation for the reaction above?
A) 1
B) 2
C) 3
D) Not enough data given to answer the question
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
5
The electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)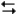 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
-Refer to Exhibit 17-3. What is the value of E°cell for the reaction above?
A) .770
B) .296
C) .7996
D) None of the above
Ag+(0.275 M) + Fe2+(0.180 M)
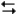 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)-Refer to Exhibit 17-3. What is the value of E°cell for the reaction above?
A) .770
B) .296
C) .7996
D) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
6
The electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)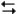 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
-Refer to Exhibit 17-3. What is the value of the equilibrium constant, K, for the reaction above at 25°C?
A) 1.15
B) 3.17
C) .142
D) Not enough information is given to answer the question
E) None of the above
Ag+(0.275 M) + Fe2+(0.180 M)
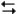 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)-Refer to Exhibit 17-3. What is the value of the equilibrium constant, K, for the reaction above at 25°C?
A) 1.15
B) 3.17
C) .142
D) Not enough information is given to answer the question
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
7
The electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)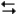 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
-Refer to Exhibit 17-3. What is the value of the reaction quotient (Q) for this reaction?
A) 4.04
B) 1.11
C) 0.727
D) 0.248
E) Not enough information is given to answer the question
F) None of the above
Ag+(0.275 M) + Fe2+(0.180 M)
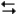 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)-Refer to Exhibit 17-3. What is the value of the reaction quotient (Q) for this reaction?
A) 4.04
B) 1.11
C) 0.727
D) 0.248
E) Not enough information is given to answer the question
F) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
8
The electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)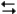 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
-Refer to Exhibit 17-3. Do you expect the silver electrode to gain mass or lose mass as the reaction approaches equilibrium?
A) Gain mass
B) Lose mass
C) Neither, solids do not appear in the equilibrium constant expression
D) Not enough information is given to answer the question
E) None of the above
Ag+(0.275 M) + Fe2+(0.180 M)
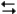 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)-Refer to Exhibit 17-3. Do you expect the silver electrode to gain mass or lose mass as the reaction approaches equilibrium?
A) Gain mass
B) Lose mass
C) Neither, solids do not appear in the equilibrium constant expression
D) Not enough information is given to answer the question
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
9
The electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)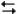 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
-Refer to Exhibit 17-3. What will be the concentration of Fe3+ when the reaction reaches equilibrium?
A) 0.183 M
B) 0.217 M
C) 0.112 M
D) 0.290 M
E) None of the above
Ag+(0.275 M) + Fe2+(0.180 M)
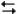 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)-Refer to Exhibit 17-3. What will be the concentration of Fe3+ when the reaction reaches equilibrium?
A) 0.183 M
B) 0.217 M
C) 0.112 M
D) 0.290 M
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
10
The electrochemical reaction below at 25°C written in standard cell notation.
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
-Refer to Exhibit 17-4. Which of the following is the chemical equation corresponding to the standard cell notation above?
A) Cd(s) + H2(g) ? Cd2+(aq) + H+(aq)
B) Cd2+(aq) + H+(aq) ? Cd(s) + H2(g)
C) Cd2+(aq) + H2(g) ? H+(aq) + H+(aq)
D) H+(aq) + H+(aq) ? Cd2+(aq) + H2(g)
E) None of the above
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
-Refer to Exhibit 17-4. Which of the following is the chemical equation corresponding to the standard cell notation above?
A) Cd(s) + H2(g) ? Cd2+(aq) + H+(aq)
B) Cd2+(aq) + H+(aq) ? Cd(s) + H2(g)
C) Cd2+(aq) + H2(g) ? H+(aq) + H+(aq)
D) H+(aq) + H+(aq) ? Cd2+(aq) + H2(g)
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
11
The electrochemical reaction below at 25°C written in standard cell notation.
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
-Refer to Exhibit 17-4. What is the value of Ecell ?
A) 0.414 V
B) 0.443 V
C) 0.662 V
D) 0.425 V
E) None of the above
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
-Refer to Exhibit 17-4. What is the value of Ecell ?
A) 0.414 V
B) 0.443 V
C) 0.662 V
D) 0.425 V
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
12
In the following equation 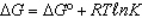
A)
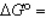 0
0
B)
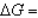
0
C) K = 1
D)
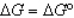
E) All of the above
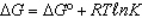
A)
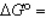 0
0B)
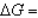
0
C) K = 1
D)
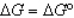
E) All of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck



