Deck 8: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/13
Play
Full screen (f)
Deck 8: Chemical Equilibrium
1
Consider the following reaction in aqueous solution:
2A + 3B + C![<strong>Consider the following reaction in aqueous solution: 2A + 3B + C D + E Which of the following is the correct law of mass action for this reaction?</strong> A) K<sub>c</sub>=[2A][3B][C][D]<sup>-</sup><sup>1</sup>[E]<sup>-</sup><sup>1</sup> B) K<sub>c</sub>=[A]<sup>2</sup>[B]<sup>3</sup>[C] \ [D][E] C) K<sub>c</sub>=[A]<sup>-</sup><sup>2</sup>[B]<sup>-</sup><sup>3</sup>[C]<sup>-</sup><sup>1</sup>[D][E] D) K<sub>c</sub>=[D][E] / [A]<sup>-</sup><sup>2</sup>[B]<sup>-</sup><sup>3</sup>[C]](https://d2lvgg3v3hfg70.cloudfront.net/TB10703/11eeda53_f154_eecb_87de_b91641bf4612_TB10703_11.jpg) D + E
D + E
Which of the following is the correct law of mass action for this reaction?
A) Kc=[2A][3B][C][D]-1[E]-1
B) Kc=[A]2[B]3[C] \ [D][E]
C) Kc=[A]-2[B]-3[C]-1[D][E]
D) Kc=[D][E] / [A]-2[B]-3[C]
2A + 3B + C
![<strong>Consider the following reaction in aqueous solution: 2A + 3B + C D + E Which of the following is the correct law of mass action for this reaction?</strong> A) K<sub>c</sub>=[2A][3B][C][D]<sup>-</sup><sup>1</sup>[E]<sup>-</sup><sup>1</sup> B) K<sub>c</sub>=[A]<sup>2</sup>[B]<sup>3</sup>[C] \ [D][E] C) K<sub>c</sub>=[A]<sup>-</sup><sup>2</sup>[B]<sup>-</sup><sup>3</sup>[C]<sup>-</sup><sup>1</sup>[D][E] D) K<sub>c</sub>=[D][E] / [A]<sup>-</sup><sup>2</sup>[B]<sup>-</sup><sup>3</sup>[C]](https://d2lvgg3v3hfg70.cloudfront.net/TB10703/11eeda53_f154_eecb_87de_b91641bf4612_TB10703_11.jpg) D + E
D + EWhich of the following is the correct law of mass action for this reaction?
A) Kc=[2A][3B][C][D]-1[E]-1
B) Kc=[A]2[B]3[C] \ [D][E]
C) Kc=[A]-2[B]-3[C]-1[D][E]
D) Kc=[D][E] / [A]-2[B]-3[C]
Kc=[A]-2[B]-3[C]-1[D][E]
2
At 25°C, Kp=2.86´1024 for the reaction:
2SO2(g) + O2(g) 2SO3(g)
2SO3(g)
At this temperature, what is the value of Kp for the reaction
SO3(g) SO2(g) +
SO2(g) + 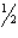 O2(g)
O2(g)
A) 1.69´1012
B) 5.91´10-13
C) 2.86´1024
D) 3.50´10-25
E) There is not enough information to answer the question
F) None of the above
2SO2(g) + O2(g)
 2SO3(g)
2SO3(g)At this temperature, what is the value of Kp for the reaction
SO3(g)
 SO2(g) +
SO2(g) + 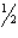 O2(g)
O2(g)A) 1.69´1012
B) 5.91´10-13
C) 2.86´1024
D) 3.50´10-25
E) There is not enough information to answer the question
F) None of the above
5.91´10-13
3
The following question(s) pertain to the equilibrium 2NO(g) + 2H2(g) N2(g) + 2H2O(g) with initial partial pressures of PNO=0.3 atm,
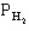 =0.4 atm,
=0.4 atm, 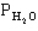 =0.4 atm.
=0.4 atm.
-Refer to Exhibit 14-1. At a certain temperature equilibrium is established and it is determined that the partial pressure of water is 0.228 atm. What are the equilibrium partial pressures of NO, H2, and N2 respectively?
A) 0.172 atm, 0.172 atm, 0.472 atm
B) 0.192 atm, 0.192 atm, 0.466 atm,
C) 0.578 atm, 0.172 atm, 0.578 atm
D) 0.114 atm, 0.114 atm, 0.472 atm
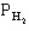 =0.4 atm,
=0.4 atm, 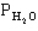 =0.4 atm.
=0.4 atm.-Refer to Exhibit 14-1. At a certain temperature equilibrium is established and it is determined that the partial pressure of water is 0.228 atm. What are the equilibrium partial pressures of NO, H2, and N2 respectively?
A) 0.172 atm, 0.172 atm, 0.472 atm
B) 0.192 atm, 0.192 atm, 0.466 atm,
C) 0.578 atm, 0.172 atm, 0.578 atm
D) 0.114 atm, 0.114 atm, 0.472 atm
0.172 atm, 0.172 atm, 0.472 atm
4
pertain to the equilibrium 2NO(g) + 2H2(g) N2(g) + 2H2O(g) with initial partial pressures of PNO=0.3 atm,
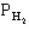 =0.4 atm,
=0.4 atm, 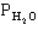 =0.4 atm.
=0.4 atm.
-Refer to Exhibit 14-1. What is the numerical value of the equilibrium constant Kp at this temperature
A) 3.63
B) .275
C) 28.0
D) .483
E) None of the above
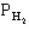 =0.4 atm,
=0.4 atm, 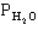 =0.4 atm.
=0.4 atm.-Refer to Exhibit 14-1. What is the numerical value of the equilibrium constant Kp at this temperature
A) 3.63
B) .275
C) 28.0
D) .483
E) None of the above

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
5
pertain to the equilibrium 2NO(g) + 2H2(g) N2(g) + 2H2O(g) with initial partial pressures of PNO=0.3 atm,
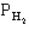 =0.4 atm,
=0.4 atm, 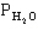 =0.4 atm.
=0.4 atm.
-Refer to Exhibit 14-1. What is the numerical value of the thermodynamic equilibrium constant K at this temperature
A) 28.0
B) 3.63
C) 1.13
D) .483
E) Not enough information is given to answer the question
F) None of the above
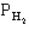 =0.4 atm,
=0.4 atm, 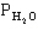 =0.4 atm.
=0.4 atm.-Refer to Exhibit 14-1. What is the numerical value of the thermodynamic equilibrium constant K at this temperature
A) 28.0
B) 3.63
C) 1.13
D) .483
E) Not enough information is given to answer the question
F) None of the above

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
6
At 100 C, the following two reactions have the denoted equilibrium constants. 
What is the value of the equilibrium constant for the reaction?
2NOBr(g) + Cl2(g)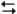 2NO(g) + 2BrCl(g)
2NO(g) + 2BrCl(g)
A) 180
B) 0.055
C) 3.125
D) .320
E) None of the above

What is the value of the equilibrium constant for the reaction?
2NOBr(g) + Cl2(g)
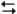 2NO(g) + 2BrCl(g)
2NO(g) + 2BrCl(g)A) 180
B) 0.055
C) 3.125
D) .320
E) None of the above

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
7
pertain to the reaction H2(g) + I2(g) 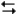 2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
-Refer to Exhibit 14-2. What is the value of Q?
A) 1.00
B) 5.00
C) 0.200
D) 0.100
E) None of the above
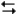 2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.-Refer to Exhibit 14-2. What is the value of Q?
A) 1.00
B) 5.00
C) 0.200
D) 0.100
E) None of the above

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
8
pertain to the reaction H2(g) + I2(g) 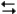 2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
-Refer to Exhibit 14-2. Based on the equilibrium constant and the value of Q, will more reactants or products be formed as the system approaches equilibrium at this temperature?
A) More products will be formed
B) More reactants will be formed
C) Not enough data to answer the question
D) None of the above
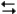 2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.-Refer to Exhibit 14-2. Based on the equilibrium constant and the value of Q, will more reactants or products be formed as the system approaches equilibrium at this temperature?
A) More products will be formed
B) More reactants will be formed
C) Not enough data to answer the question
D) None of the above

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
9
pertain to the reaction H2(g) + I2(g) 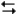 2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
-Refer to Exhibit 14-2. What will be the partial pressure of HI when the system reaches equilibrium?
A) 0.200 atm
B) 0.037 atm
C) 0.526 atm
D) 0.373 atm
E) Not enough data to answer the question
F) None of the above
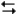 2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.-Refer to Exhibit 14-2. What will be the partial pressure of HI when the system reaches equilibrium?
A) 0.200 atm
B) 0.037 atm
C) 0.526 atm
D) 0.373 atm
E) Not enough data to answer the question
F) None of the above

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
10
pertain to the reaction N2(g) + 3H2(g) 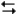 2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
-Refer to Exhibit 14-3. What is the value of the equilibrium constant Kp at this temperature?
A) 0.652
B) 2.78´10-21
C) 3.59´1020
D) 1.92´10-14
E) None of the above
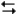 2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.-Refer to Exhibit 14-3. What is the value of the equilibrium constant Kp at this temperature?
A) 0.652
B) 2.78´10-21
C) 3.59´1020
D) 1.92´10-14
E) None of the above

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
11
pertain to the reaction N2(g) + 3H2(g) 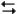 2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
-Refer to Exhibit 14-3. Based on the equilibrium constant and the value of Q, will more reactants or products be formed as the system approaches equilibrium at this temperature?
A) More products will be formed
B) More reactants will be formed
C) Not enough data to answer the question
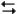 2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.-Refer to Exhibit 14-3. Based on the equilibrium constant and the value of Q, will more reactants or products be formed as the system approaches equilibrium at this temperature?
A) More products will be formed
B) More reactants will be formed
C) Not enough data to answer the question

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
12
pertain to the reaction N2(g) + 3H2(g) 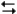 2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
-Refer to Exhibit 14-3. What will be the total pressure in the reaction vessel when the system reaches equilibrium at this temperature?
A) 1.24 atm
B) 0.900 atm
C) 0.744 atm
D) 0.305 atm
E) None of the above
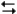 2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.-Refer to Exhibit 14-3. What will be the total pressure in the reaction vessel when the system reaches equilibrium at this temperature?
A) 1.24 atm
B) 0.900 atm
C) 0.744 atm
D) 0.305 atm
E) None of the above

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
13
At room temperature cyclohexane exists almost exclusively in the chair conformation (99.99%). But at 800°C, 30% of the cyclohexane molecules exist in the twist-boat conformation.
Compared to its value at 25°C, the value of DG° at 800°C for the reaction
C6H12(chair)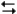 C6H12(twist-boat)
C6H12(twist-boat)
Should
A) be larger in magnitude and have the same sign
B) be larger in magnitude and have the opposite sign
C) be smaller in magnitude and have the same sign
D) be smaller in magnitude and have the opposite sign
E) not enough information to answer the question
Compared to its value at 25°C, the value of DG° at 800°C for the reaction
C6H12(chair)
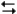 C6H12(twist-boat)
C6H12(twist-boat)Should
A) be larger in magnitude and have the same sign
B) be larger in magnitude and have the opposite sign
C) be smaller in magnitude and have the same sign
D) be smaller in magnitude and have the opposite sign
E) not enough information to answer the question

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck



