Deck 2: Chemical Formulas, Chemical Equations, and Reaction Yields
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/15
Play
Full screen (f)
Deck 2: Chemical Formulas, Chemical Equations, and Reaction Yields
1
What is the relative molecular mass of the compound trinitrotoluene, C7H5N3O6 (on the 12C scale)?
A) 43.03
B) 205.13
C) 215.13
D) 227.13
E) 278.03
A) 43.03
B) 205.13
C) 215.13
D) 227.13
E) 278.03
227.13
2
Vitamin B12, cyanocobalamin, has the molecular formula, C68H88CoN14O14P. What is the percent mass of cobalt in this compound?
A) 1.02%
B) 4.35%
C) 10.3%
D) 22.3%
E) 23.2%
A) 1.02%
B) 4.35%
C) 10.3%
D) 22.3%
E) 23.2%
4.35%
3
For the given unbalanced reaction
X CaO(s) + y H2O(l) z Ca(OH)2
The correct stiochiometric coefficients x, y, & z are
A) 1,1,1
B) 1,2,1
C) 2,1,2
D) 2,1,1
E) 1,1,2
X CaO(s) + y H2O(l) z Ca(OH)2
The correct stiochiometric coefficients x, y, & z are
A) 1,1,1
B) 1,2,1
C) 2,1,2
D) 2,1,1
E) 1,1,2
1,1,1
4
In fermentation, sucrose, C12H22O11, reacts with water to form ethanol, C2H5OH, and carbon dioxide. A balanced chemical equation for this reaction is?
A) C12H22O11(aq) + 3 H2O(l) 5 C2H5OH(aq) + 2 Co2(g)
B) C12H22O11(aq) + 7 H2O(l) 2 C2H5OH(aq) + 8 Co2(g)
C) C12H22O11(aq) + 2 H2O(l) 6 C2H5OH(aq) + Co2(g)
D) C12H22O11(aq) + 2 H2O(l) 4 C2H5OH(aq) + 4 Co2(g)
E) C12H22O11(aq) + H2O(l) 4 C2H5OH(aq) + 4 Co2(g)
A) C12H22O11(aq) + 3 H2O(l) 5 C2H5OH(aq) + 2 Co2(g)
B) C12H22O11(aq) + 7 H2O(l) 2 C2H5OH(aq) + 8 Co2(g)
C) C12H22O11(aq) + 2 H2O(l) 6 C2H5OH(aq) + Co2(g)
D) C12H22O11(aq) + 2 H2O(l) 4 C2H5OH(aq) + 4 Co2(g)
E) C12H22O11(aq) + H2O(l) 4 C2H5OH(aq) + 4 Co2(g)

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
5
Calcium carbide, CaC2, reacts with water to produce calcium hydroxide and acetylene gas, C2H2.
CaC2(s) + 2 H2O(l) Ca(OH)2(aq) + C2H2(g)
How many grams of acetylene can be produced from 30.0 g of calcium carbide?
A) 1.42 g
B) 12.2 g
C) 28.0 g
D) 30.0 g
E) 64.1 g
CaC2(s) + 2 H2O(l) Ca(OH)2(aq) + C2H2(g)
How many grams of acetylene can be produced from 30.0 g of calcium carbide?
A) 1.42 g
B) 12.2 g
C) 28.0 g
D) 30.0 g
E) 64.1 g

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
6
Ammonium perchlorate, NH4ClO4, is used with aluminum as rocket fuel.
10 Al(s) + 6 NH4ClO4 4 Al2O3 + 2 AlCl3 + 12 H2O + 3 N2
If 100.0 g of aluminum are reacted with 60.0 g of ammonium perchlorate, how many grams of aluminum oxide are produced?
A) 34.71 g
B) 40.00 g
C) 123.8 g
D) 151.1 g
E) 1789 g
10 Al(s) + 6 NH4ClO4 4 Al2O3 + 2 AlCl3 + 12 H2O + 3 N2
If 100.0 g of aluminum are reacted with 60.0 g of ammonium perchlorate, how many grams of aluminum oxide are produced?
A) 34.71 g
B) 40.00 g
C) 123.8 g
D) 151.1 g
E) 1789 g

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
7
Aluminum sulfide reacts with water to form aluminum hydroxide and hydrogen sulfide by the following reaction
Al2S3 + 6 H2O 2Al(OH)3 + 3H2S
If 25 g of aluminum sulfide is reacted with 25 g of water how many moles of hydrogen sulfide will be formed?
A) 0.12 moles
B) 0.50 moles
C) 0.78 moles
D) 2.0 moles
E) 3.0 moles
Al2S3 + 6 H2O 2Al(OH)3 + 3H2S
If 25 g of aluminum sulfide is reacted with 25 g of water how many moles of hydrogen sulfide will be formed?
A) 0.12 moles
B) 0.50 moles
C) 0.78 moles
D) 2.0 moles
E) 3.0 moles

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
8
Iron oxide can be reduced to iron by a reaction with carbon to form carbon monoxide
Fe2O3(s) + 3C(s) 2Fe(s) + 3CO(g)
If 95.0 grams of iron oxide is reacted with excess carbon yields 63 g of iron, what is the percent yield of this reaction?
A) 12%
B) 59%
C) 66%
D) 95%
E) 100%
Fe2O3(s) + 3C(s) 2Fe(s) + 3CO(g)
If 95.0 grams of iron oxide is reacted with excess carbon yields 63 g of iron, what is the percent yield of this reaction?
A) 12%
B) 59%
C) 66%
D) 95%
E) 100%

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
9
Acetonitrile, CH3CN, can be synthesized from carbon monoxide, hydrogen, and ammonia in the presence of a catalyst at high temperatures by the following reaction
2CO(g) + 2H2(g)+NH3(g) CH3CN(g) + 2H2O(g)
If 20 g of carbon monoxide, 20 g of hydrogen, and 10 g of ammonia are reacted, which will be the limiting reagent?
A) carbon monoxide
B) hydrogen
C) ammonia
D) none of them
E) there is no way to know
2CO(g) + 2H2(g)+NH3(g) CH3CN(g) + 2H2O(g)
If 20 g of carbon monoxide, 20 g of hydrogen, and 10 g of ammonia are reacted, which will be the limiting reagent?
A) carbon monoxide
B) hydrogen
C) ammonia
D) none of them
E) there is no way to know

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
10
Which has a larger molar volume (the volume occupied by one mole):
Gold (density = 19.3 g cm-3) or tin (density = 7.31 g cm-3)?
A) gold
B) tin
C) they are exactly the same
D) there is no way to know without other information
Gold (density = 19.3 g cm-3) or tin (density = 7.31 g cm-3)?
A) gold
B) tin
C) they are exactly the same
D) there is no way to know without other information

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
11
Copper oxide ore, CuO, can be smelted with carbon to make copper metal and carbon dioxide.
2CuO(s) + C(s) 2Cu(s) + CO2(g)
If 100.0 of a mixed ore is smelted and produces 75.9 grams of pure copper. What percentage of the mixed ore is CuO? (You can assume the reaction yield is 100%, there is excess carbon, and that CuO is the only source of copper.)
A) 28.0%
B) 48.5%
C) 75.9%
D) 79.9%
E) 95.0%
2CuO(s) + C(s) 2Cu(s) + CO2(g)
If 100.0 of a mixed ore is smelted and produces 75.9 grams of pure copper. What percentage of the mixed ore is CuO? (You can assume the reaction yield is 100%, there is excess carbon, and that CuO is the only source of copper.)
A) 28.0%
B) 48.5%
C) 75.9%
D) 79.9%
E) 95.0%

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
12
A vanadium oxide contains 56.02% vanadium by mass. What is its empirical formula?
A) V2O
B) VO
C) V2O3
D) VO2
E) V2O5
A) V2O
B) VO
C) V2O3
D) VO2
E) V2O5

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
13
Caffeine has the molecular formula, C8H10N4O2. If it is burned in excess oxygen it will form carbon dioxide, water vapor, and nitrogen gas. Which gas will be most abundant by mass?
A) CO2
B) H2O
C) N2
D) they will all the be the same
E) it depends on the mass of caffeine
A) CO2
B) H2O
C) N2
D) they will all the be the same
E) it depends on the mass of caffeine

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
14
Triphenylene is an organic compound containing only carbon and hydrogen with 3 carbon atoms for every two hydrogen atoms. Which of the following is a possible molecular mass for triphenylene?
A) 34.0 g/mol
B) 228.29 g/mol
C) 366.8 g/mol
D) 400.0 g/mol
E) b or c could be correct
A) 34.0 g/mol
B) 228.29 g/mol
C) 366.8 g/mol
D) 400.0 g/mol
E) b or c could be correct

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
15
Sodium perbromate can be synthesized via the following reaction that gives off Xenon gas.
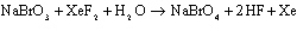
A reaction is started with 200.0g of NaBrO3, 250.0g of XeF2 and 100.0g of water. Assuming that the reaction goes to completion, and that all of the formed xenon gas is allowed to escape into the atmosphere, what will be the minimum mass of products and reactants remaining in the reaction vessel?
A) 173.4 g
B) 193.9 g
C) 376.0 g
D) 356.1 g
E) None of the above
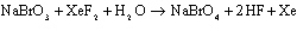
A reaction is started with 200.0g of NaBrO3, 250.0g of XeF2 and 100.0g of water. Assuming that the reaction goes to completion, and that all of the formed xenon gas is allowed to escape into the atmosphere, what will be the minimum mass of products and reactants remaining in the reaction vessel?
A) 173.4 g
B) 193.9 g
C) 376.0 g
D) 356.1 g
E) None of the above

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck



