Deck 3: An Introduction to Organic Reactions and Their Mechanisms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/13
Play
Full screen (f)
Deck 3: An Introduction to Organic Reactions and Their Mechanisms
1
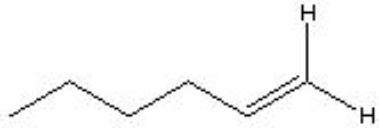
What type of reaction is shown below? 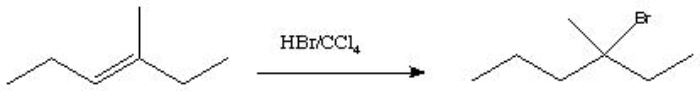
A) addition
B) substitution
C) elimination
D) rearrangement

A) addition
B) substitution
C) elimination
D) rearrangement
addition
2
In the following acid-base reaction, the indicated molecule is a(n) ___ ? 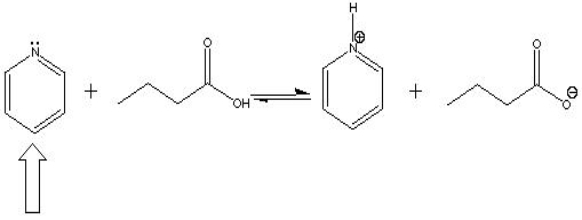
A) Acid
B) Base
C) Conjugate acid
D) Conjugate base

A) Acid
B) Base
C) Conjugate acid
D) Conjugate base
Base
3
In the following acid-base reaction, the indicated molecule is a(n) ___ ? 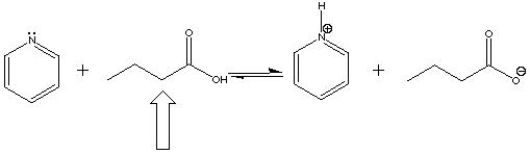
A) Acid
B) Base
C) Conjugate acid
D) Conjugate base

A) Acid
B) Base
C) Conjugate acid
D) Conjugate base
Acid
4
In the following acid-base reaction, the indicated molecule is a(n) ___ . 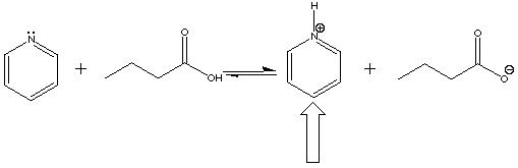
A) Acid
B) Base
C) Conjugate acid
D) Conjugate base

A) Acid
B) Base
C) Conjugate acid
D) Conjugate base

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
5
In the following acid-base reaction, the indicated molecule is a(n) ___ . 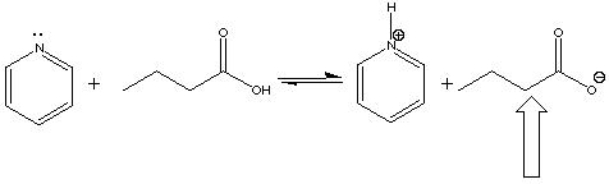
A) Acid
B) Base
C) Conjugate acid
D) Conjugate base

A) Acid
B) Base
C) Conjugate acid
D) Conjugate base

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following structures are Lewis Acids? 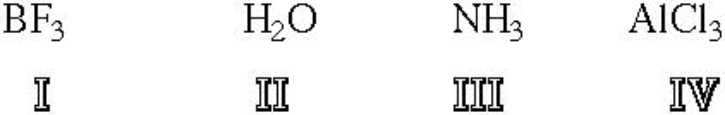
A) I
B) II
C) III
D) IV
E) I and IV
F) II and III
G) I, III and IV
A) I
B) II
C) III
D) IV
E) I and IV
F) II and III
G) I, III and IV

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following structures are Lewis Bases?
A) I
B) II
C) III
D) IV
E) I and IV
F) II and IV
G) II, III and IV
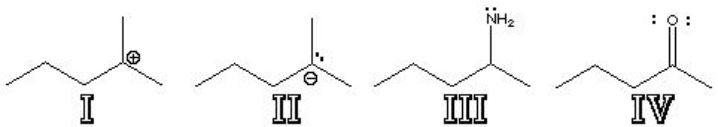
A) I
B) II
C) III
D) IV
E) I and IV
F) II and IV
G) II, III and IV


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
8
Using pKa table 3.1, page 111, what is the strongest base?
A) H2O
B) Br-
C) NH3
D) OH-
E) CH3CH2-
A) H2O
B) Br-
C) NH3
D) OH-
E) CH3CH2-

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
9
Using pKa table 3.1, page 111, which base below is needed to deprotonate the following structure?
A) CH3CH2-
B) F-
C) NH2-
D) OH-
E) C6H5O-
A) CH3CH2-
B) F-
C) NH2-
D) OH-
E) C6H5O-


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
10
Using the periodic chart, which is the strongest bas?
A) C-
B) N-
C) O-
D) F-
A) C-
B) N-
C) O-
D) F-

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
11
Using the periodic chart, which will have the highest pKa?
A) Br-
B) Cl-
C) F-
D) I-
A) Br-
B) Cl-
C) F-
D) I-

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
12
Using the periodic chart, which is the strongest acid?
A) H2S
B) HCl
C) H3P
D) SiH4
A) H2S
B) HCl
C) H3P
D) SiH4

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is the most acidic? 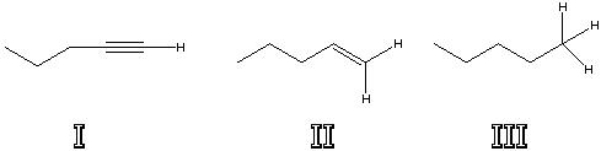
A) I
B) II
C) III

A) I
B) II
C) III

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck



