Deck 2: Essential Chemistry for Biology
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/42
Play
Full screen (f)
Deck 2: Essential Chemistry for Biology
1
All atoms of an element have the same number of ______.
A) protons plus neutrons
B) protons
C) electrons
D) neutrons
A) protons plus neutrons
B) protons
C) electrons
D) neutrons
B
2
The four most common elements found in living things are
A) nitrogen, oxygen, phosphorus, and carbon.
B) carbon, oxygen, nitrogen, and hydrogen.
C) carbon, oxygen, potassium, and calcium.
D) oxygen, calcium, hydrogen, and carbon.
A) nitrogen, oxygen, phosphorus, and carbon.
B) carbon, oxygen, nitrogen, and hydrogen.
C) carbon, oxygen, potassium, and calcium.
D) oxygen, calcium, hydrogen, and carbon.
B
3
An atom with a positive charge has ______.
A) more protons than electrons
B) more electrons than protons
C) more neutrons than protons
D) more protons than neutrons
A) more protons than electrons
B) more electrons than protons
C) more neutrons than protons
D) more protons than neutrons
A
4
An atom with an electrical charge is a(n)______.
A) isotope
B) molecule
C) ion
D) compound
A) isotope
B) molecule
C) ion
D) compound

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
5
The hydrogens and oxygen of a water molecule are held together by ______ bonds.
A) electron
B) hydrogen
C) covalent
D) osmotic
A) electron
B) hydrogen
C) covalent
D) osmotic

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
6
An uncharged atom of gold has an atomic number of 79 and an atomic mass of 197.This atom has ______ protons,______ neutrons,and ______ electrons.
A) 79... 118... 79
B) 118... 79... 118
C) 118... 276... 118
D) 79... 34... 79
A) 79... 118... 79
B) 118... 79... 118
C) 118... 276... 118
D) 79... 34... 79

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
7
The way Earth moves about the sun is most like ______.
A) a neutron and electron moving around a proton
B) an electron moving around the nucleus of an atom
C) a proton moving about an electron
D) a neutron moving about a proton
A) a neutron and electron moving around a proton
B) an electron moving around the nucleus of an atom
C) a proton moving about an electron
D) a neutron moving about a proton

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
8
What name is given to bonds that involve the sharing of electrons?
A) covalent
B) hydrogen
C) ionic
D) polar
A) covalent
B) hydrogen
C) ionic
D) polar

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
9
The second electron shell of an atom can hold a maximum of ______ electron(s).
A) 1
B) 2
C) 6
D) 8
A) 1
B) 2
C) 6
D) 8

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
10
Beryllium's atomic mass is 9 and its atomic number is 4.How many neutrons are found in a beryllium atom?
A) 9
B) 13
C) 4
D) 5
A) 9
B) 13
C) 4
D) 5

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
11
How do radioactive isotopes differ from isotopes?
A) Radioactive isotopes have more neutrons than do isotopes.
B) Radioactive isotopes are stable; isotopes are unstable.
C) Radioactive isotopes have fewer neutrons than do isotopes.
D) Radioactive isotopes are unstable; isotopes are stable.
A) Radioactive isotopes have more neutrons than do isotopes.
B) Radioactive isotopes are stable; isotopes are unstable.
C) Radioactive isotopes have fewer neutrons than do isotopes.
D) Radioactive isotopes are unstable; isotopes are stable.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
12
Nitrogen has an atomic number of 7; therefore,it has ______ electrons in its outermost electron shell.
A) 10
B) 18
C) 5
D) 2
A) 10
B) 18
C) 5
D) 2

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
13
Why is water considered a polar molecule?
A) The oxygen is found between the two hydrogens.
B) The oxygen atom attracts the hydrogen atoms.
C) The oxygen end of the molecule has a slight negative charge, and the hydrogen end has a slight positive charge.
D) Both hydrogens are at one end of the molecule, and oxygen is at the other end.
A) The oxygen is found between the two hydrogens.
B) The oxygen atom attracts the hydrogen atoms.
C) The oxygen end of the molecule has a slight negative charge, and the hydrogen end has a slight positive charge.
D) Both hydrogens are at one end of the molecule, and oxygen is at the other end.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
14
An atom's ______ are found in its nucleus.
A) neutrons and protons
B) protons only
C) neutrons and electrons
D) electrons, protons, and neutrons
A) neutrons and protons
B) protons only
C) neutrons and electrons
D) electrons, protons, and neutrons

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
15
______ is an example of an element.
A) Water
B) Carbon
C) Glucose
D) Salt
A) Water
B) Carbon
C) Glucose
D) Salt

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
16
In the following reaction,what type of bond is holding the two atoms together? K + Cl → K+ + Cl- → KCl
A) hydrophilic
B) ionic
C) hydrophobic
D) covalent
A) hydrophilic
B) ionic
C) hydrophobic
D) covalent

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
17
The bond between oppositely charged ions is a(n)______ bond.
A) ionic
B) polar
C) hydrogen
D) covalent
A) ionic
B) polar
C) hydrogen
D) covalent

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
18
Sulfur has an atomic number of 16.How many covalent bonds can sulfur form?
A) 1
B) 2
C) 4
D) 0
A) 1
B) 2
C) 4
D) 0

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
19
Isotopes of an element have the same number of ______ and different numbers of ______.
A) protons... neutrons
B) protons... electrons
C) neutrons... protons
D) electrons... protons
A) protons... neutrons
B) protons... electrons
C) neutrons... protons
D) electrons... protons

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following elements,essential to life,is a trace element?
A) phosphorus
B) carbon
C) iodine
D) calcium
A) phosphorus
B) carbon
C) iodine
D) calcium

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
21
Relative to a pH of 6,a pH of 4 has a ______.
A) 200 times higher H+ concentration
B) 100 times higher H+ concentration
C) 20 times higher H+ concentration
D) 100 times lower H+ concentration
A) 200 times higher H+ concentration
B) 100 times higher H+ concentration
C) 20 times higher H+ concentration
D) 100 times lower H+ concentration

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
22
People have long speculated about whether life exists on Mars.Scientists have evidence that on Mars,______.
A) microbial life exists
B) liquid water has existed in the past
C) the only water present has always been frozen in the polar ice caps
D) water is found only in the form of water vapor
A) microbial life exists
B) liquid water has existed in the past
C) the only water present has always been frozen in the polar ice caps
D) water is found only in the form of water vapor

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
23
The tendency of molecules of the same kind to stick together is called ______.
A) bonding
B) cohesion
C) polarity
D) adhesion
A) bonding
B) cohesion
C) polarity
D) adhesion

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
24
A base ______.
A) removes H2O molecules from a solution
B) decreases the pH of a solution
C) removes OH- ions from a solution
D) removes H+ ions from a solution
A) removes H2O molecules from a solution
B) decreases the pH of a solution
C) removes OH- ions from a solution
D) removes H+ ions from a solution

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
25
What are the reactant(s)in the following chemical reaction? C6H12O6 + 6 H2O + 6 O2 → 6 CO2 + 12 H2O
A) CO2 and H2O
B) C6H12O6, H2O, and O2
C) O2 only
D) C6H12O6, H2O, O2, CO2, and H2O
A) CO2 and H2O
B) C6H12O6, H2O, and O2
C) O2 only
D) C6H12O6, H2O, O2, CO2, and H2O

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
26
Sugar dissolves when stirred into water.The sugar is the ______,the water is the ______,and the sweetened water is the ______.
A) solution... solvent... solute
B) solute... solvent... solution
C) solvent... solute... solution
D) solution... solute... solvent
A) solution... solvent... solute
B) solute... solvent... solution
C) solvent... solute... solution
D) solution... solute... solvent

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
27
Adjacent water molecules are joined by ______ bonds.
A) covalent only
B) ionic
C) polar and covalent
D) hydrogen
A) covalent only
B) ionic
C) polar and covalent
D) hydrogen

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
28
Adjacent water molecules are connected by the ______.
A) sharing of electrons between the hydrogen of one water molecule and the oxygen of another water molecule
B) electrical attraction between the hydrogen of one water molecule and the oxygen of another water molecule
C) sharing of electrons between adjacent oxygen molecules
D) electrical attraction between the hydrogens of adjacent water molecules
A) sharing of electrons between the hydrogen of one water molecule and the oxygen of another water molecule
B) electrical attraction between the hydrogen of one water molecule and the oxygen of another water molecule
C) sharing of electrons between adjacent oxygen molecules
D) electrical attraction between the hydrogens of adjacent water molecules

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is an acid?
A) NaOH
B) NaCl
C) HCl
D) CH4
A) NaOH
B) NaCl
C) HCl
D) CH4

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
30
Why (if you are careful)are you able to float a needle on the surface of water?
A) Water has adhesive properties.
B) The surface tension that is a result of water's cohesive properties makes this possible.
C) The covalent bonds that hold a water molecule together are responsible for this ability.
D) A single needle is less dense than water.
A) Water has adhesive properties.
B) The surface tension that is a result of water's cohesive properties makes this possible.
C) The covalent bonds that hold a water molecule together are responsible for this ability.
D) A single needle is less dense than water.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
31
How many oxygen atoms are in the products of the following reaction? C6H12O6 + 6 H2O + 6 O2 → 6 CO2 + 12 H2O
A) 18
B) 6
C) 12
D) 24
A) 18
B) 6
C) 12
D) 24

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
32
Examine the pH scale below.How does household bleach compare to household ammonia? 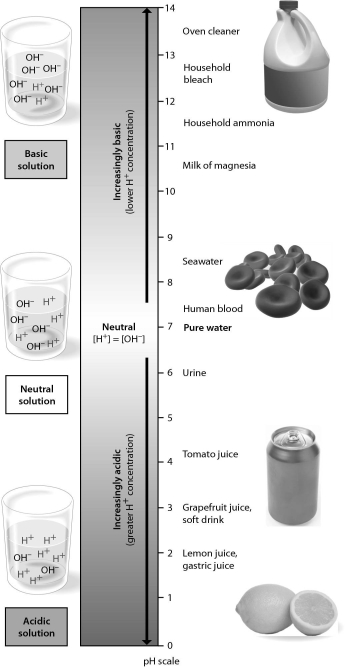
A) Household bleach is more acidic than household ammonia.
B) Household bleach has 10 times higher H+ concentration than household ammonia.
C) Household bleach has 100 times higher H+ concentration than household ammonia.
D) Household ammonia has 10 times higher H+ concentration.

A) Household bleach is more acidic than household ammonia.
B) Household bleach has 10 times higher H+ concentration than household ammonia.
C) Household bleach has 100 times higher H+ concentration than household ammonia.
D) Household ammonia has 10 times higher H+ concentration.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
33
Examine the following figure.Which of the representations of molecules does not reveal double bonds? 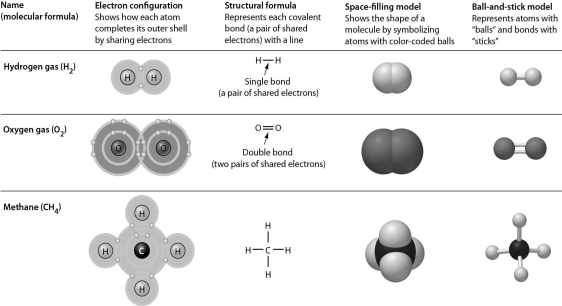
A) electron configuration
B) structural formula
C) space-filling model
D) All of the representations of molecules reveal double bonds.

A) electron configuration
B) structural formula
C) space-filling model
D) All of the representations of molecules reveal double bonds.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
34
Sweating cools your body by ______.
A) cohesion
B) radiation
C) evaporative cooling
D) hydrogen bonding
A) cohesion
B) radiation
C) evaporative cooling
D) hydrogen bonding

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
35
Examine the drawing of an atom below.The art is technically incorrect in that ______. 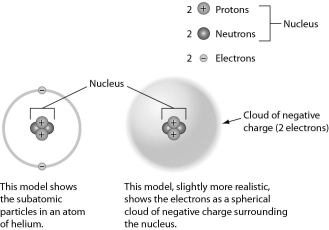
A) neutrons are not located in the nucleus
B) the electrons should be much farther away from the nucleus
C) electrons do not orbit the nucleus
D) electrons do not have a negative charge

A) neutrons are not located in the nucleus
B) the electrons should be much farther away from the nucleus
C) electrons do not orbit the nucleus
D) electrons do not have a negative charge

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
36
The lower the pH of a solution,the ______.
A) greater the number of oxygen atoms
B) more acidic the solution
C) less toxic the solution
D) higher the OH- concentration
A) greater the number of oxygen atoms
B) more acidic the solution
C) less toxic the solution
D) higher the OH- concentration

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
37
Human body cells are approximately ______ water.
A) 95-99%
B) 25-35%
C) 50-55%
D) 70-95%
A) 95-99%
B) 25-35%
C) 50-55%
D) 70-95%

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
38
When a base is added to a buffered solution,the buffer will ______.
A) donate OH- ions
B) accept water molecules
C) donate H+ ions
D) form covalent bonds with the base
A) donate OH- ions
B) accept water molecules
C) donate H+ ions
D) form covalent bonds with the base

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
39
As water freezes,______.
A) its molecules move farther apart
B) it cools the surrounding environment
C) its hydrogen bonds break apart
D) it loses its polarity
A) its molecules move farther apart
B) it cools the surrounding environment
C) its hydrogen bonds break apart
D) it loses its polarity

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
40
What name is given to substances that resist changes in pH?
A) buffers
B) sugars
C) salts
D) bases
A) buffers
B) sugars
C) salts
D) bases

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
41
Please read the following scenario to answer the following question(s).
The last few miles of the marathon are the most difficult for Heather. Her hair is plastered to her head, sweat clings to her arms, and her legs feel as if they had nothing left. Heather grabs a cup of ice water. The ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. Then a breeze kicks up and she finally feels some coolness against her skin. Drops of sweat, once clinging to her forehead, now spill down, and Heather feels a stinging as the sweat flows into her eyes.
Which of the following is the most likely reason why the ice struck Heather's nose when she took a drink?
A) Water can store large amounts of heat.
B) Water can moderate temperatures through evaporative cooling.
C) The density of water decreases when it freezes.
D) Water has a cohesive nature.
The last few miles of the marathon are the most difficult for Heather. Her hair is plastered to her head, sweat clings to her arms, and her legs feel as if they had nothing left. Heather grabs a cup of ice water. The ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. Then a breeze kicks up and she finally feels some coolness against her skin. Drops of sweat, once clinging to her forehead, now spill down, and Heather feels a stinging as the sweat flows into her eyes.
Which of the following is the most likely reason why the ice struck Heather's nose when she took a drink?
A) Water can store large amounts of heat.
B) Water can moderate temperatures through evaporative cooling.
C) The density of water decreases when it freezes.
D) Water has a cohesive nature.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
42
Please read the following scenario to answer the following question(s).
The last few miles of the marathon are the most difficult for Heather. Her hair is plastered to her head, sweat clings to her arms, and her legs feel as if they had nothing left. Heather grabs a cup of ice water. The ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. Then a breeze kicks up and she finally feels some coolness against her skin. Drops of sweat, once clinging to her forehead, now spill down, and Heather feels a stinging as the sweat flows into her eyes.
Sweat on Heather's forehead and arms formed drops because of the ______.
A) high salt content of sweat
B) cohesive nature of water
C) ability of water to moderate heat
D) high evaporative cooling effect of water
The last few miles of the marathon are the most difficult for Heather. Her hair is plastered to her head, sweat clings to her arms, and her legs feel as if they had nothing left. Heather grabs a cup of ice water. The ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. Then a breeze kicks up and she finally feels some coolness against her skin. Drops of sweat, once clinging to her forehead, now spill down, and Heather feels a stinging as the sweat flows into her eyes.
Sweat on Heather's forehead and arms formed drops because of the ______.
A) high salt content of sweat
B) cohesive nature of water
C) ability of water to moderate heat
D) high evaporative cooling effect of water

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck



