Deck 2: Basic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 2: Basic Chemistry
1
An acid is a molecule that releases (donates) ________.
A) protons (hydrogen ions)
B) hydroxyl ions
C) neutrons
D) electrons
A) protons (hydrogen ions)
B) hydroxyl ions
C) neutrons
D) electrons
A
2
An atom with 13 electrons will have ________ electrons in the valence shell.
A) 2
B) 3
C) 5
D) 8
A) 2
B) 3
C) 5
D) 8
B
3
Polar molecules, like water, result when electrons are shared ________.
A) unequally between atoms
B) between ions
C) equally between atoms
D) or transferred between atoms
A) unequally between atoms
B) between ions
C) equally between atoms
D) or transferred between atoms
A
4
Nerve impulses involve the flow of an electrical current, a type of energy known as ________ energy.
A) radiant
B) mechanical
C) electrical
D) chemical
A) radiant
B) mechanical
C) electrical
D) chemical

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
During a synthesis reaction, amino acids join to form ________.
A) carbohydrates
B) proteins
C) monomers
D) nucleic acids
A) carbohydrates
B) proteins
C) monomers
D) nucleic acids

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
A nucleotide of DNA contain three components: ________, ________, and ________.
A) deoxyribose; a phosphate group; nitrogen-containing base
B) ribose; three phosphate groups; nitrogen-containing base
C) ribose; two phosphate groups; acid group
D) ribose; a phosphate group; nitrogen-containing base
A) deoxyribose; a phosphate group; nitrogen-containing base
B) ribose; three phosphate groups; nitrogen-containing base
C) ribose; two phosphate groups; acid group
D) ribose; a phosphate group; nitrogen-containing base

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
Matter is best described as ________.
A) having no mass
B) the ability to put matter into motion
C) anything that occupies space and has mass
D) the ability to do work
A) having no mass
B) the ability to put matter into motion
C) anything that occupies space and has mass
D) the ability to do work

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
Using Figure 2.1, identify the following:
Letter E represents a nucleic acid building block known as a ________.
A) monosaccharide
B) triglyceride
C) saturated fat
D) nucleotide

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
Enzymes are examples of ________ proteins.
A) structural
B) globular (functional)
C) fibrous
D) alpha
A) structural
B) globular (functional)
C) fibrous
D) alpha

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
The most common element in the human body is ________.
A) carbon
B) oxygen
C) hydrogen
D) nitrogen
A) carbon
B) oxygen
C) hydrogen
D) nitrogen

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
The atomic number of an atom is equal to the number of ________ an atom contains.
A) protons
B) neutrons
C) protons and neutrons
D) neutrons and electrons
A) protons
B) neutrons
C) protons and neutrons
D) neutrons and electrons

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
The complementary base to adenine in a molecule of DNA is ________.
A) guanine
B) cytosine
C) leucine
D) thymine
A) guanine
B) cytosine
C) leucine
D) thymine

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13

Using Figure 2.1, identify the following:
Letter D represents the structure of a(n) ________.
A) monosaccharide
B) amino acid
C) triglyceride
D) steroid

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
Glycogen and starch are examples of a specific category of carbohydrates called ________.
A) monosaccharides
B) triglycerides
C) steroids
D) polysaccharides
A) monosaccharides
B) triglycerides
C) steroids
D) polysaccharides

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
Unsaturated fatty acid chains contain one or more ________ bonds between carbon atoms.
A) peptide
B) double
C) triple
D) monosaccharide
A) peptide
B) double
C) triple
D) monosaccharide

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
The pH scale is based on the number of ________ in solution.
A) neutrons
B) electrons
C) protons
D) hydroxyls
A) neutrons
B) electrons
C) protons
D) hydroxyls

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17

Using Figure 2.1, identify the following:
Which letter represents a globular protein in its quaternary structure?
A) Label A
B) Label B
C) Label C
D) Label D
E) Label E

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is NOT a subatomic particle associated with an atom?
A) neutron
B) electron
C) proton
D) ion
A) neutron
B) electron
C) proton
D) ion

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19

Using Figure 2.1, identify the following:
Which letter represents a carbohydrate polymer?
A) Label A
B) Label B
C) Label C
D) Label D
E) Label E

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
Which property of water explains its ability to prevent sudden changes in body temperature?
A) cushioning
B) chemical reactant
C) polarity
D) high heat capacity
A) cushioning
B) chemical reactant
C) polarity
D) high heat capacity

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
The atomic number of sodium is 11 while the atomic mass number is 23. Which of the following is NOT True of an atom of sodium?
A) 11 protons
B) 8 electrons in the valence shell of a neutral sodium atom
C) 11 neutrons
D) 11 electrons
E) 1 electron in the valence shell of a neutral sodium atom
A) 11 protons
B) 8 electrons in the valence shell of a neutral sodium atom
C) 11 neutrons
D) 11 electrons
E) 1 electron in the valence shell of a neutral sodium atom

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
The number of protons always equals the ________ in a neutral atom.
A) atomic mass number
B) number of electrons
C) number of neutrons
D) atomic weight
E) number of valence shells
A) atomic mass number
B) number of electrons
C) number of neutrons
D) atomic weight
E) number of valence shells

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
The growth of cells and repair of worn-out tissues is accomplished by ________.
A) decomposition reactions
B) catabolic reactions
C) hydrolysis reactions
D) synthesis reactions
E) neutralization reactions
A) decomposition reactions
B) catabolic reactions
C) hydrolysis reactions
D) synthesis reactions
E) neutralization reactions

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following contains sodium?
A) H₂O
B) NaCl
C) N₂
D) CH₄
E) H₂SO₄
A) H₂O
B) NaCl
C) N₂
D) CH₄
E) H₂SO₄

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
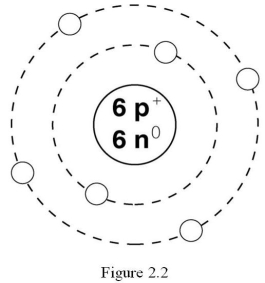
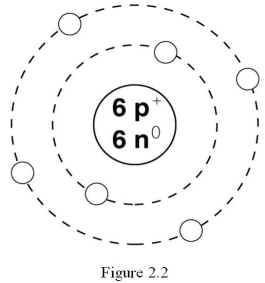
What is the atomic number of the atom in Figure 2.2?
A) 2
B) 3
C) 4
D) 6
E) 12

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
The subatomic particles that are responsible for the chemical behavior of atoms are the ________.
A) protons
B) neutrons
C) electrons
D) isotopes
E) ions
A) protons
B) neutrons
C) electrons
D) isotopes
E) ions

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
A molecule of methane, CH₄, is known specifically as a(n) ________.
A) compound
B) radioisotope
C) element
D) atom
E) anion
A) compound
B) radioisotope
C) element
D) atom
E) anion

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
Elements are composed of building blocks known as ________.
A) molecules
B) atoms
C) compounds
D) polymers
E) protons
A) molecules
B) atoms
C) compounds
D) polymers
E) protons

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
In order to break a disaccharide down into simple sugar units, ________.
A) water molecules must be added to each bond
B) water molecules must be removed from each bond
C) carbon atoms must be added to each bond
D) carbon atoms must be removed from each bond
E) water molecules and carbon atoms must be removed from each bond
A) water molecules must be added to each bond
B) water molecules must be removed from each bond
C) carbon atoms must be added to each bond
D) carbon atoms must be removed from each bond
E) water molecules and carbon atoms must be removed from each bond

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
Isotopes have different numbers of ________; thus they also have different ________.
A) protons; atomic numbers
B) neutrons; atomic masses
C) electrons; atomic numbers
D) protons; atomic masses
E) neutrons; atomic numbers
A) protons; atomic numbers
B) neutrons; atomic masses
C) electrons; atomic numbers
D) protons; atomic masses
E) neutrons; atomic numbers

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
The movement of ions across plasma membranes is an example of ________.
A) radiant energy
B) chemical energy
C) electrical energy
D) mechanical energy
E) potential energy
A) radiant energy
B) chemical energy
C) electrical energy
D) mechanical energy
E) potential energy

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following leads to an increase in the rate of a chemical reaction?
A) increased temperature
B) large particle size
C) lack of catalysts
D) decreased temperature
E) few particles
A) increased temperature
B) large particle size
C) lack of catalysts
D) decreased temperature
E) few particles

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
An atom with 6 protons, 7 neutrons, and 6 electrons shares four pairs of electrons with four other atoms. This atom is now considered to be ________.
A) a cation
B) an anion
C) a neutral atom
D) stable
E) an ion
A) a cation
B) an anion
C) a neutral atom
D) stable
E) an ion

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following elements is needed to make a functional hemoglobin molecule?
A) magnesium
B) iodine
C) iron
D) potassium
E) chlorine
A) magnesium
B) iodine
C) iron
D) potassium
E) chlorine

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
An atom of magnesium has lost two electrons. It is known as a(n) ________.
A) anion
B) molecule
C) isotope
D) cation
E) neutral atom
A) anion
B) molecule
C) isotope
D) cation
E) neutral atom

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
An atom has 6 protons, 8 neutrons, and 6 electrons. Its atomic mass number is ________.
A) 2
B) 6
C) 8
D) 14
E) 20
A) 2
B) 6
C) 8
D) 14
E) 20

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is classified as an inorganic compound?
A) glucose
B) triglyceride
C) water
D) protein
E) steroid
A) glucose
B) triglyceride
C) water
D) protein
E) steroid

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
The atomic mass number of an atom is equivalent to the number of ________.
A) protons
B) neutrons
C) electrons
D) protons and electrons
E) protons and neutrons
A) protons
B) neutrons
C) electrons
D) protons and electrons
E) protons and neutrons

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following may be used in PET scans as biological tracers that can be followed through the body?
A) nucleic acids
B) proteins
C) electrons
D) ions
E) radioisotopes
A) nucleic acids
B) proteins
C) electrons
D) ions
E) radioisotopes

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
What type of bond results when electrons are completely transferred from one atom to another?
A) ionic bond
B) hydrogen bond
C) carbon bond
D) polar covalent bond
E) nonpolar covalent bond
A) ionic bond
B) hydrogen bond
C) carbon bond
D) polar covalent bond
E) nonpolar covalent bond

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
The reaction sucrose + water → glucose + fructose is an example of a(n) ________.
A) double replacement reaction
B) synthesis reaction
C) decomposition reaction
D) neutralization reaction
E) anabolic reaction
A) double replacement reaction
B) synthesis reaction
C) decomposition reaction
D) neutralization reaction
E) anabolic reaction

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
Blood pH falls in a narrow range between ________.
A) 7.0 to 8.0
B) 6.0 to 8.0
C) 7.35 to 7.45
D) 7.15 to 7.25
E) 7.65 to 7.85
A) 7.0 to 8.0
B) 6.0 to 8.0
C) 7.35 to 7.45
D) 7.15 to 7.25
E) 7.65 to 7.85

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
Which of these vitamins is produced in skin upon exposure to ultraviolet (UV) radiation?
A) vitamin A
B) vitamin C
C) vitamin D
D) vitamin E
E) vitamin K
A) vitamin A
B) vitamin C
C) vitamin D
D) vitamin E
E) vitamin K

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
Which statement best describes why ATP is an important nucleic acid in the body?
A) ATP is the storage form of glucose in the body.
B) ATP is a modified RNA molecule used to store genetic information.
C) ATP carries out the orders for protein synthesis issued by DNA.
D) ATP functions as a catalyst to increase reaction rates.
E) ATP provides a form of chemical energy all body cells can use.
A) ATP is the storage form of glucose in the body.
B) ATP is a modified RNA molecule used to store genetic information.
C) ATP carries out the orders for protein synthesis issued by DNA.
D) ATP functions as a catalyst to increase reaction rates.
E) ATP provides a form of chemical energy all body cells can use.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
Which polysaccharide is formed of linked glucose molecules and stored in animal tissues?
A) ribose
B) cellulose
C) starch
D) glucose
E) glycogen
A) ribose
B) cellulose
C) starch
D) glucose
E) glycogen

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
The building blocks of a triglyceride are ________.
A) three fatty acid chains and one glycerol molecule
B) one fatty acid chain and one glycerol molecule
C) four interlocking rings of carbon and hydrogen atoms
D) amino acids
E) nucleotides
A) three fatty acid chains and one glycerol molecule
B) one fatty acid chain and one glycerol molecule
C) four interlocking rings of carbon and hydrogen atoms
D) amino acids
E) nucleotides

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following lipids is formed of four interlocking carbon rings?
A) phospholipid
B) cholesterol
C) triglyceride
D) trans fat
E) unsaturated fat
A) phospholipid
B) cholesterol
C) triglyceride
D) trans fat
E) unsaturated fat

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following DNA base pairs is complementary?
A) adenine and guanine
B) guanine and uracil
C) thymine and guanine
D) cytosine and adenine
E) adenine and thymine
A) adenine and guanine
B) guanine and uracil
C) thymine and guanine
D) cytosine and adenine
E) adenine and thymine

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
Enzymes are ________.
A) carbohydrates
B) stable at high temperatures
C) biological catalysts
D) not reusable
E) required in large amounts in order to be effective
A) carbohydrates
B) stable at high temperatures
C) biological catalysts
D) not reusable
E) required in large amounts in order to be effective

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
Hydrogen bonding between water molecules is responsible for ________.
A) polarity
B) denaturation of proteins
C) enzyme structure
D) nonpolar covalent bonding
E) surface tension
A) polarity
B) denaturation of proteins
C) enzyme structure
D) nonpolar covalent bonding
E) surface tension

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
Two or more polypeptides chains combine to form a complex structure called a ________.
A) primary structure
B) beta-pleated sheet
C) secondary structure
D) tertiary structure
E) quaternary structure
A) primary structure
B) beta-pleated sheet
C) secondary structure
D) tertiary structure
E) quaternary structure

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
Exchange reactions in which an acid and a base interact are known as ________.
A) decomposition reactions
B) neutralization reactions
C) anabolic reactions
D) hydrolysis reactions
E) catabolic reactions
A) decomposition reactions
B) neutralization reactions
C) anabolic reactions
D) hydrolysis reactions
E) catabolic reactions

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the nucleic acid.
A) oxidase
B) cholesterol
C) glucose
D) DNA
E) triglyceride
A) oxidase
B) cholesterol
C) glucose
D) DNA
E) triglyceride

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following solutions is the weakest acid?
A) a solution with a pH of 2.4
B) a solution with a pH of 5.2
C) a solution with a pH of 6.4
D) a solution with a pH of 8.6
E) a solution with a pH of 10.1
A) a solution with a pH of 2.4
B) a solution with a pH of 5.2
C) a solution with a pH of 6.4
D) a solution with a pH of 8.6
E) a solution with a pH of 10.1

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
Saturated fats ________.
A) have two fatty acid chains
B) exist as solids at room temperature
C) are formed from four interlocking carbon rings
D) contain many double bonds
E) exist as liquids and are derived from plants
A) have two fatty acid chains
B) exist as solids at room temperature
C) are formed from four interlocking carbon rings
D) contain many double bonds
E) exist as liquids and are derived from plants

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
Which carbohydrate is also known as blood sugar?
A) sucrose
B) glucose
C) ribose
D) deoxyribose
E) cellulose
A) sucrose
B) glucose
C) ribose
D) deoxyribose
E) cellulose

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following statements about RNA is True?
A) RNA is a single nucleotide strand.
B) RNA is composed of the bases cytosine, guanine, adenine, and thymine.
C) RNA is found only in the nucleus of the cell.
D) RNA contains a sugar called deoxyribose.
E) RNA is the genetic material found within the cell nucleus.
A) RNA is a single nucleotide strand.
B) RNA is composed of the bases cytosine, guanine, adenine, and thymine.
C) RNA is found only in the nucleus of the cell.
D) RNA contains a sugar called deoxyribose.
E) RNA is the genetic material found within the cell nucleus.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
Which statement best describes fibrous proteins?
A) Fibrous proteins are the major source of stored energy in the body.
B) Fibrous proteins most often appear in body structures.
C) Fibrous proteins are the basis for all body steroids.
D) Fibrous proteins are spherical molecules.
E) Fibrous proteins are considered water-soluble proteins.
A) Fibrous proteins are the major source of stored energy in the body.
B) Fibrous proteins most often appear in body structures.
C) Fibrous proteins are the basis for all body steroids.
D) Fibrous proteins are spherical molecules.
E) Fibrous proteins are considered water-soluble proteins.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
A chemist experiments on a molecule with the formula of C₅H₁₀O₅. This compound is likely a(n) ________.
A) protein
B) triglyceride
C) enzyme
D) carbohydrate
E) nucleotide
A) protein
B) triglyceride
C) enzyme
D) carbohydrate
E) nucleotide

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
The organic compounds that function in building tissues and acting as enzymes are the ________.
A) nucleic acids
B) carbohydrates
C) salts
D) lipids
E) proteins
A) nucleic acids
B) carbohydrates
C) salts
D) lipids
E) proteins

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
Hydrogen bonds are very strong bonds that hold together water molecules.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
Electrolytes conduct electrical currents in solution.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
Inorganic compounds lack carbon and tend to be small, simple molecules.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is classified as a protein?
A) cholesterol
B) enzyme
C) glucose
D) triglyceride
E) RNA
A) cholesterol
B) enzyme
C) glucose
D) triglyceride
E) RNA

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
A molecule with the sequence of AUCGUCA should be categorized as ________.
A) ATP
B) an enzyme
C) RNA
D) DNA
E) a triglyceride
A) ATP
B) an enzyme
C) RNA
D) DNA
E) a triglyceride

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
Inactive or stored energy is called kinetic energy.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following would be classified as an enzyme?
A) hydrolase
B) cholesterol
C) triglyceride
D) cellulose
E) ATP
A) hydrolase
B) cholesterol
C) triglyceride
D) cellulose
E) ATP

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
The lower the pH, the greater the number of hydrogen ions released by a chemical into solution.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following statements about ATP is false?
A) ATP drives the transport of certain solutes (e.g., amino acids) across cell membranes.
B) ATP activates contractile proteins in muscle cells so that cells can shorten and perform mechanical work.
C) ATP provides the energy needed to drive energy-absorbing chemical reactions.
D) ATP is a modified nucleotide.
E) The phosphate groups of ATP are attached by high-energy hydrogen bonds.
A) ATP drives the transport of certain solutes (e.g., amino acids) across cell membranes.
B) ATP activates contractile proteins in muscle cells so that cells can shorten and perform mechanical work.
C) ATP provides the energy needed to drive energy-absorbing chemical reactions.
D) ATP is a modified nucleotide.
E) The phosphate groups of ATP are attached by high-energy hydrogen bonds.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
The four most common elements in the human body are oxygen, carbon, nitrogen, and hydrogen.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
What type of chemical bond is pictured in Figure 2.3?
A) nonpolar covalent bond
B) polar covalent bond
C) ionic bond
D) single covalent bond
E) double covalent bond
A) nonpolar covalent bond
B) polar covalent bond
C) ionic bond
D) single covalent bond
E) double covalent bond

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
The number of protons in an atom equals the atomic number for that element.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
Atoms that have lost or gained electrons during chemical bonding are known as isotopes.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
The building blocks of proteins are ________.
A) monosaccharides
B) nucleotides
C) amino acids
D) nucleic acids
E) fatty acids
A) monosaccharides
B) nucleotides
C) amino acids
D) nucleic acids
E) fatty acids

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
Neutralization reactions that occur between an acid and a base are a type of exchange reaction.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
Shell 1 of an atom can hold a maximum of ________ electron(s).
A) 1
B) 2
C) 4
D) 8
E) 18
A) 1
B) 2
C) 4
D) 8
E) 18

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
Water is the single most abundant inorganic compound in the human body.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
The nucleotide chains of DNA are held together by ________.
A) carbon bonds
B) hydrogen bonds
C) ionic bonds
D) nonpolar covalent bonds
E) polar covalent bonds
A) carbon bonds
B) hydrogen bonds
C) ionic bonds
D) nonpolar covalent bonds
E) polar covalent bonds

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
What is the function of DNA?
A) DNA provides instructions for building every protein in the body.
B) DNA serves as a form of chemical energy that all body cells can use.
C) DNA serves as the most important fuel for body cells.
D) DNA carries out the orders for protein synthesis issued by RNA.
E) DNA increases the rate of a chemical reaction without becoming part of the product.
A) DNA provides instructions for building every protein in the body.
B) DNA serves as a form of chemical energy that all body cells can use.
C) DNA serves as the most important fuel for body cells.
D) DNA carries out the orders for protein synthesis issued by RNA.
E) DNA increases the rate of a chemical reaction without becoming part of the product.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
Trans fats are oils that have been solidified by the addition of ________.
A) oxygen atoms
B) carbon atoms
C) hydrogen atoms
D) nitrogen atoms
E) phosphorus-containing groups
A) oxygen atoms
B) carbon atoms
C) hydrogen atoms
D) nitrogen atoms
E) phosphorus-containing groups

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck



