Deck 2: Small Molecules and the Chemistry of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/246
Play
Full screen (f)
Deck 2: Small Molecules and the Chemistry of Life
1
According to the periodic table, the compound that sulfur forms with hydrogen is most like
A) NH4+.
B) NH3.
C) H2O.
D) HF.
E) HCl.
A) NH4+.
B) NH3.
C) H2O.
D) HF.
E) HCl.
C
2
Carbon and silicon have the same number of
A) protons.
B) valence (outer shell) electrons.
C) neutrons.
D) electrons.
E) protons and neutrons.
A) protons.
B) valence (outer shell) electrons.
C) neutrons.
D) electrons.
E) protons and neutrons.
B
3
A lithium atom contains three protons.For this atom to remain inert in an electric field, it must also contain
A) three neutrons.
B) three electrons.
C) two neutrons and two electrons.
D) no electrons.
E) no neutrons.
A) three neutrons.
B) three electrons.
C) two neutrons and two electrons.
D) no electrons.
E) no neutrons.
B
4
According to the periodic table, which element has the same number of outer shell (valence) electrons as oxygen?
A) Calcium
B) Nitrogen
C) Fluorine
D) Sodium
E) Sulfur
A) Calcium
B) Nitrogen
C) Fluorine
D) Sodium
E) Sulfur

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
5
An atom with _______ has an atomic mass of 14.
A) 14 neutrons
B) 14 electrons
C) 7 neutrons and 7 electrons
D) 7 protons and 7 electrons
E) 6 protons and 8 neutrons
A) 14 neutrons
B) 14 electrons
C) 7 neutrons and 7 electrons
D) 7 protons and 7 electrons
E) 6 protons and 8 neutrons

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
6
A stable isotope of phosphorus has an atomic number of 15 and an atomic mass of 31.How many neutrons does this isotope of phosphorus have?
A) 14
B) 16
C) 30
D) 31
E) 46
A) 14
B) 16
C) 30
D) 31
E) 46

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
7
The best reference source for the atomic number and mass number of an element is
A) a good chemistry text.
B) a dictionary.
C) the periodic table.
D) a general physics book.
E) a good biology text.
A) a good chemistry text.
B) a dictionary.
C) the periodic table.
D) a general physics book.
E) a good biology text.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement about an atom is true?
A) Only protons contribute significantly to the atom's mass.
B) Only neutrons contribute significantly to the atom's mass.
C) Only electrons contribute significantly to the atom's mass.
D) Both protons and neutrons together contribute significantly to the atom's mass.
E) Both protons and electrons together contribute significantly to the atom's mass.
A) Only protons contribute significantly to the atom's mass.
B) Only neutrons contribute significantly to the atom's mass.
C) Only electrons contribute significantly to the atom's mass.
D) Both protons and neutrons together contribute significantly to the atom's mass.
E) Both protons and electrons together contribute significantly to the atom's mass.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
9
The six elements most common in organisms are
A) calcium, iron, hydrogen, phosphorus, potassium, and oxygen.
B) water, carbon, hydrogen, nitrogen, sodium, and oxygen.
C) carbon, oxygen, hydrogen, phosphorus, sulfur, and nitrogen.
D) nitrogen, carbon, iron, sulfur, calcium, and hydrogen.
E) phosphorus, helium, carbon, potassium, hydrogen, and oxygen.
A) calcium, iron, hydrogen, phosphorus, potassium, and oxygen.
B) water, carbon, hydrogen, nitrogen, sodium, and oxygen.
C) carbon, oxygen, hydrogen, phosphorus, sulfur, and nitrogen.
D) nitrogen, carbon, iron, sulfur, calcium, and hydrogen.
E) phosphorus, helium, carbon, potassium, hydrogen, and oxygen.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
10
Which element has a higher atomic mass than phosphorus?
A) Hydrogen
B) Oxygen
C) Sodium
D) Magnesium
E) Calcium
A) Hydrogen
B) Oxygen
C) Sodium
D) Magnesium
E) Calcium

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
11
In the history of the discovery of the parts of an atom, the neutron was discovered after the proton and electron.What property of a neutron made it more difficult than the proton or electron to discover?
A) Diameter
B) Location in the nucleus
C) Mass
D) Lack of charge
E) Presence in isotopes
A) Diameter
B) Location in the nucleus
C) Mass
D) Lack of charge
E) Presence in isotopes

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
12
The number of different natural elements found in the universe is closest to
A) 18.
B) 54.
C) 86.
D) 94.
E) 146.
A) 18.
B) 54.
C) 86.
D) 94.
E) 146.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
13
The number of protons in a neutral atom equals the number of
A) electrons.
B) neutrons.
C) electrons plus neutrons.
D) neutrons minus electrons.
E) isotopes.
A) electrons.
B) neutrons.
C) electrons plus neutrons.
D) neutrons minus electrons.
E) isotopes.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
14
Which pair has similar chemical properties?
A) 12C and 14C
B) 12C and 40Ca
C) 16O and 16N
D) 1H and 22Na
E) 18O and 45Ca
A) 12C and 14C
B) 12C and 40Ca
C) 16O and 16N
D) 1H and 22Na
E) 18O and 45Ca

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
15
The mass number of an atom is determined primarily by the _______ it contains.
A) number of electrons
B) number of protons
C) sum of the number of protons and the number of electrons
D) sum of the number of protons and the number of neutrons
E) number of charges
A) number of electrons
B) number of protons
C) sum of the number of protons and the number of electrons
D) sum of the number of protons and the number of neutrons
E) number of charges

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
16
What is the difference between an atom and an element?
A) An atom is made of protons, electrons, and (most of the time) neutrons; an element is composed of only one kind of atom.
B) An element is made of protons, electrons, and (most of the time) neutrons; an atom is composed of only one kind of element.
C) An atom does not contain electrons, whereas an element does.
D) An atom contains protons and electrons, whereas an element contains protons, electrons, and neutrons.
E) All atoms are the same, whereas elements differ in structure and properties.
A) An atom is made of protons, electrons, and (most of the time) neutrons; an element is composed of only one kind of atom.
B) An element is made of protons, electrons, and (most of the time) neutrons; an atom is composed of only one kind of element.
C) An atom does not contain electrons, whereas an element does.
D) An atom contains protons and electrons, whereas an element contains protons, electrons, and neutrons.
E) All atoms are the same, whereas elements differ in structure and properties.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
17
The atomic number of an element is the same as the number of _______ in each atom.
A) neutrons
B) neutrons plus electrons
C) neutrons plus protons
D) protons
E) protons plus electrons
A) neutrons
B) neutrons plus electrons
C) neutrons plus protons
D) protons
E) protons plus electrons

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements about the atom is true?
A) There are usually more protons than electrons in an atom because the negative charge of an electron is larger than the positive charge of a proton.
B) The negative charge of an electron adds mass to an atom without influencing other properties.
C) In an atom with a neutral charge, the number of electrons is equal to the number of protons.
D) The number of electrons determines whether an atom of an element is radioactive.
E) The energy level of electrons is higher in shells close to the nucleus of the atom.
A) There are usually more protons than electrons in an atom because the negative charge of an electron is larger than the positive charge of a proton.
B) The negative charge of an electron adds mass to an atom without influencing other properties.
C) In an atom with a neutral charge, the number of electrons is equal to the number of protons.
D) The number of electrons determines whether an atom of an element is radioactive.
E) The energy level of electrons is higher in shells close to the nucleus of the atom.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
19
Refer to the table below. 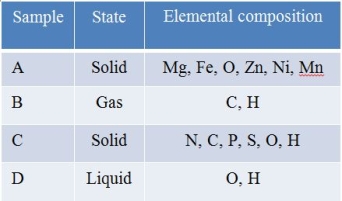 Four samples taken from an underground geologic site were analyzed in a chemistry lab.The table summarizes the elements found in greatest abundance in each sample.Which sample(s) could have originated from living sources?
Four samples taken from an underground geologic site were analyzed in a chemistry lab.The table summarizes the elements found in greatest abundance in each sample.Which sample(s) could have originated from living sources?
A) C only
B) A and B
C) B and C
D) A and C
E) B, C, and D
 Four samples taken from an underground geologic site were analyzed in a chemistry lab.The table summarizes the elements found in greatest abundance in each sample.Which sample(s) could have originated from living sources?
Four samples taken from an underground geologic site were analyzed in a chemistry lab.The table summarizes the elements found in greatest abundance in each sample.Which sample(s) could have originated from living sources?A) C only
B) A and B
C) B and C
D) A and C
E) B, C, and D

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
20
Carbon-12 is the most abundant isotope of carbon on Earth.Carbon-13 makes up about 1 percent of Earth's carbon atoms and is useful for radio imaging.Which of the following is true?
A) Carbon-13 has more protons than carbon-12.
B) Carbon-13 has more neutrons than carbon-12.
C) Carbon-13 has more electrons than carbon-12.
D) Carbon-13 has an electronic configuration that is different from that of carbon-12.
E) Carbon-13 has an equal number of protons and neutrons.
A) Carbon-13 has more protons than carbon-12.
B) Carbon-13 has more neutrons than carbon-12.
C) Carbon-13 has more electrons than carbon-12.
D) Carbon-13 has an electronic configuration that is different from that of carbon-12.
E) Carbon-13 has an equal number of protons and neutrons.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
21
All of the elements listed below follow the octet rule except
A) hydrogen.
B) chlorine.
C) carbon.
D) sodium.
E) nitrogen.
A) hydrogen.
B) chlorine.
C) carbon.
D) sodium.
E) nitrogen.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
22
The two covalent bonds in a water molecule are polar because
A) oxygen is more electronegative than hydrogen.
B) oxygen and hydrogen have similar electronegativities.
C) oxygen is less electronegative than hydrogen.
D) water is a small molecule.
E) water is hydrophilic.
A) oxygen is more electronegative than hydrogen.
B) oxygen and hydrogen have similar electronegativities.
C) oxygen is less electronegative than hydrogen.
D) water is a small molecule.
E) water is hydrophilic.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
23
Which atom usually has the greatest number of covalent bonds with other atoms?
A) Carbon
B) Oxygen
C) Sulfur
D) Hydrogen
E) Nitrogen
A) Carbon
B) Oxygen
C) Sulfur
D) Hydrogen
E) Nitrogen

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement about ionic and covalent bonds is true?
A) An ionic bond is stronger than a covalent bond.
B) Compared with an ionic bond, a nonpolar covalent bond has more equal electron sharing.
C) An ionic bond is almost identical to a nonpolar covalent bond.
D) Ionic bonds vary in length, but covalent bonds are all the same length.
E) An ionic bond can have multiple bonds, but a covalent bond cannot.
A) An ionic bond is stronger than a covalent bond.
B) Compared with an ionic bond, a nonpolar covalent bond has more equal electron sharing.
C) An ionic bond is almost identical to a nonpolar covalent bond.
D) Ionic bonds vary in length, but covalent bonds are all the same length.
E) An ionic bond can have multiple bonds, but a covalent bond cannot.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
25
In a hydrogen molecule, the two atoms are held together by
A) hydrogen bonds.
B) a shared pair of electrons.
C) van der Waals forces.
D) ionic attractions.
E) differences in electronegativity.
A) hydrogen bonds.
B) a shared pair of electrons.
C) van der Waals forces.
D) ionic attractions.
E) differences in electronegativity.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
26
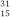 P and
P and 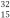 P have virtually identical chemical and biological properties because they have the same
P have virtually identical chemical and biological properties because they have the sameA) half-life.
B) number of neutrons.
C) atomic weight.
D) mass number.
E) number of electrons.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
27
A double covalent chemical bond represents the sharing of _______ electron(s).
A) one
B) two
C) three
D) four
E) six
A) one
B) two
C) three
D) four
E) six

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the elements listed below requires two additional electrons to fill the outermost electron shell?
A) Lithium
B) Carbon
C) Nitrogen
D) Oxygen
E) Fluorine
A) Lithium
B) Carbon
C) Nitrogen
D) Oxygen
E) Fluorine

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
29
Oxygen forms _______ covalent bond(s), carbon forms _______, and hydrogen forms _______.
A) one; four; one
B) four; four; four
C) two; four; none
D) two; four; one
E) two; two; two
A) one; four; one
B) four; four; four
C) two; four; none
D) two; four; one
E) two; two; two

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
30
The ability of an atom to combine with other atoms is determined by the atom's
A) atomic weight.
B) ability to form isomers.
C) number and distribution of electrons.
D) nuclear configuration.
E) mass number.
A) atomic weight.
B) ability to form isomers.
C) number and distribution of electrons.
D) nuclear configuration.
E) mass number.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
31
Which statement is true?
A) Carbon makes the same number of covalent bonds as phosphorus does.
B) Oxygen makes more covalent bonds than sulfur does.
C) Sulfur makes more covalent bonds than carbon does.
D) Hydrogen makes more covalent bonds than carbon does.
E) Oxygen makes fewer covalent bonds than nitrogen does.
A) Carbon makes the same number of covalent bonds as phosphorus does.
B) Oxygen makes more covalent bonds than sulfur does.
C) Sulfur makes more covalent bonds than carbon does.
D) Hydrogen makes more covalent bonds than carbon does.
E) Oxygen makes fewer covalent bonds than nitrogen does.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
32
Refer to the Bohr model of methane shown below. 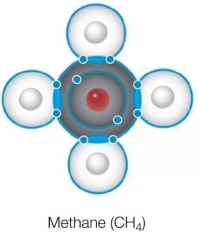 Which statement about this structure is true?
Which statement about this structure is true?
A) All bonds are ionic bonds.
B) All bonds are hydrogen bonds.
C) All bonds contain paired electrons from carbon.
D) All bonds contain paired electrons from hydrogen.
E) All bonds contain paired electrons shared between carbon and hydrogen.
 Which statement about this structure is true?
Which statement about this structure is true?A) All bonds are ionic bonds.
B) All bonds are hydrogen bonds.
C) All bonds contain paired electrons from carbon.
D) All bonds contain paired electrons from hydrogen.
E) All bonds contain paired electrons shared between carbon and hydrogen.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
33
Which correctly shows the relative strengths of chemical bonds in decreasing order?
A) Covalent, ionic, hydrogen, van der Waals forces
B) Ionic, covalent, hydrogen, van der Waals forces
C) van der Waals forces, covalent, ionic, hydrogen
D) Hydrogen, covalent, van der Waals forces, ionic
E) Ionic, covalent, van der Waals forces, hydrogen
A) Covalent, ionic, hydrogen, van der Waals forces
B) Ionic, covalent, hydrogen, van der Waals forces
C) van der Waals forces, covalent, ionic, hydrogen
D) Hydrogen, covalent, van der Waals forces, ionic
E) Ionic, covalent, van der Waals forces, hydrogen

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
34
Two carbon atoms held together in a double covalent bond share _______ electron(s).
A) one
B) two
C) four
D) six
E) eight
A) one
B) two
C) four
D) six
E) eight

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
35
What part of the atom determines how the atom reacts chemically?
A) Proton
B) Neutron
C) Electron
D) Innermost shell
E) Nucleus
A) Proton
B) Neutron
C) Electron
D) Innermost shell
E) Nucleus

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
36
Nitrogen-14 and nitrogen-15 are isotopes.Nitrogen-15 is used to determine protein structure.Which of the following is true?
A) Nitrogen-15 has more neutrons than nitrogen-14.
B) Nitrogen-15 has more protons than nitrogen-14.
C) Nitrogen-15 has more electrons than nitrogen-14.
D) Nitrogen-15 has an electronic configuration that is different from that of nitrogen-14.
E) Nitrogen-15 has an equal number of protons and neutrons.
A) Nitrogen-15 has more neutrons than nitrogen-14.
B) Nitrogen-15 has more protons than nitrogen-14.
C) Nitrogen-15 has more electrons than nitrogen-14.
D) Nitrogen-15 has an electronic configuration that is different from that of nitrogen-14.
E) Nitrogen-15 has an equal number of protons and neutrons.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
37
An atom is most stable when
A) it can have one unpaired valence electron, allowing it to follow the octet rule.
B) it can share electrons with other atoms to form an uneven number of pairs of electrons.
C) it has eight electrons.
D) it can fill its outermost shell by sharing electrons or by gaining or losing one or more electrons until it is filled.
E) its outermost electron shell follows the quartet rule.
A) it can have one unpaired valence electron, allowing it to follow the octet rule.
B) it can share electrons with other atoms to form an uneven number of pairs of electrons.
C) it has eight electrons.
D) it can fill its outermost shell by sharing electrons or by gaining or losing one or more electrons until it is filled.
E) its outermost electron shell follows the quartet rule.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
38
Which element is the most chemically reactive?
A) Carbon
B) Helium
C) Neon
D) Argon
E) Krypton
A) Carbon
B) Helium
C) Neon
D) Argon
E) Krypton

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
39
Drawings of hydrogen, deuterium, and tritium would contain different numbers of
A) protons.
B) neutrons.
C) electrons.
D) nuclei.
E) electron shells.
A) protons.
B) neutrons.
C) electrons.
D) nuclei.
E) electron shells.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
40
Differences in the electronegativity of atoms that share electrons in a bond are involved in
A) a polar covalent bond.
B) an ionic bond.
C) a hydrogen bond.
D) van der Waals forces.
E) hydrophobic interactions.
A) a polar covalent bond.
B) an ionic bond.
C) a hydrogen bond.
D) van der Waals forces.
E) hydrophobic interactions.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
41
A covalent bond is formed by the sharing of _______ between atoms, whereas an ionic bond is formed by the _______.
A) neutrons; sharing of electrons
B) electrons; electric attraction between two neutral atoms
C) protons; electric attraction between two neutral atoms
D) protons; sharing of electrons
E) electrons; transfer of electrons from one atom to another
A) neutrons; sharing of electrons
B) electrons; electric attraction between two neutral atoms
C) protons; electric attraction between two neutral atoms
D) protons; sharing of electrons
E) electrons; transfer of electrons from one atom to another

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
42
Carbon-14 is a radioactive isotope of carbon.When an organism is alive, the total amount of carbon-14 in the organism's body remains constant.As soon as the organism dies, the amount of carbon-14 begins to decrease in a predictable way.This provides evidence for which statement about living organisms?
A) Different organisms have different life-spans.
B) Living organisms are dynamic and constantly exchanging matter with the environment.
C) There is a huge diversity of life-forms represented among the organisms living today.
D) All living organisms are composed of cells.
E) Living organisms pass on biological information to their offspring.
A) Different organisms have different life-spans.
B) Living organisms are dynamic and constantly exchanging matter with the environment.
C) There is a huge diversity of life-forms represented among the organisms living today.
D) All living organisms are composed of cells.
E) Living organisms pass on biological information to their offspring.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
43
Which statement about biochemical reactions is false?
A) They obey the rules of chemistry and physics.
B) They must always balance the number of atoms in the reactants and the products.
C) They can create new energy during the reaction.
D) They can store energy in the form of a covalent bond.
E) They can change the form of energy found in the cell.
A) They obey the rules of chemistry and physics.
B) They must always balance the number of atoms in the reactants and the products.
C) They can create new energy during the reaction.
D) They can store energy in the form of a covalent bond.
E) They can change the form of energy found in the cell.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
44
Refer to the figure below. 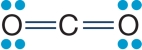 The figure shows the molecular structure of carbon dioxide.Carbon dioxide is nonpolar, whereas water is polar.Which of the true statements below explains these differences?
The figure shows the molecular structure of carbon dioxide.Carbon dioxide is nonpolar, whereas water is polar.Which of the true statements below explains these differences?
A) Carbon dioxide does not contain any polar covalent bonds, whereas water does.
B) Carbon dioxide contains only double bonds, whereas water contains only single bonds.
C) Carbon dioxide is a linear molecule, whereas water has a bent shape.
D) Carbon dioxide contains carbon atoms, whereas water does not.
E) Carbon and oxygen do not differ greatly in electronegativity, whereas hydrogen and oxygen do.
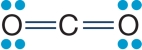 The figure shows the molecular structure of carbon dioxide.Carbon dioxide is nonpolar, whereas water is polar.Which of the true statements below explains these differences?
The figure shows the molecular structure of carbon dioxide.Carbon dioxide is nonpolar, whereas water is polar.Which of the true statements below explains these differences?A) Carbon dioxide does not contain any polar covalent bonds, whereas water does.
B) Carbon dioxide contains only double bonds, whereas water contains only single bonds.
C) Carbon dioxide is a linear molecule, whereas water has a bent shape.
D) Carbon dioxide contains carbon atoms, whereas water does not.
E) Carbon and oxygen do not differ greatly in electronegativity, whereas hydrogen and oxygen do.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
45
Refer to the oxidation-reduction reaction below.
Fe + Cu2+ Cu + Fe2+
This reaction occurs spontaneously when a strip of iron metal is placed into a solution of copper sulfate dissolved in water.During the reaction, iron metal is oxidized to form a cation, and copper ion is reduced to form copper metal.Which statement describes the change taking place?
A) The change is not a chemical change because no covalent bonds were broken and new ones formed.
B) The change is not a chemical change because there were too few elements involved.
C) The change is a chemical change because the products differ chemically from the reactants.
D) The change is a chemical change because it occurred spontaneously.
E) The change is a chemical change because there was no overall change in mass.
Fe + Cu2+ Cu + Fe2+
This reaction occurs spontaneously when a strip of iron metal is placed into a solution of copper sulfate dissolved in water.During the reaction, iron metal is oxidized to form a cation, and copper ion is reduced to form copper metal.Which statement describes the change taking place?
A) The change is not a chemical change because no covalent bonds were broken and new ones formed.
B) The change is not a chemical change because there were too few elements involved.
C) The change is a chemical change because the products differ chemically from the reactants.
D) The change is a chemical change because it occurred spontaneously.
E) The change is a chemical change because there was no overall change in mass.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
46
A chemist measures the masses of two substances separately, then combines them in a reaction flask and heats the mixture.After several minutes, the chemist cools the flask and measures the mass of the contents.The final mass of the contents is less than the sum of the masses of the two substances placed in the flask before heating.Which statement provides a possible explanation for this observation?
A) Physical changes in the two starting substances resulted in products with less combined mass than the starting substances.
B) Heating caused the substances to melt, which resulted in a change in overall volume and mass.
C) The two starting substances absorbed energy from the heat, which destroyed some of the atoms making up the substances.
D) Only one product was formed from the combination of two reactants, resulting in less overall mass at the end.
E) The two starting substances underwent chemical change to produce two products, one of which was a gas.
A) Physical changes in the two starting substances resulted in products with less combined mass than the starting substances.
B) Heating caused the substances to melt, which resulted in a change in overall volume and mass.
C) The two starting substances absorbed energy from the heat, which destroyed some of the atoms making up the substances.
D) Only one product was formed from the combination of two reactants, resulting in less overall mass at the end.
E) The two starting substances underwent chemical change to produce two products, one of which was a gas.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
47
All of the following are nonpolar except
A) O2.
B) N2.
C) CH4.
D) NaCl.
E) H2.
A) O2.
B) N2.
C) CH4.
D) NaCl.
E) H2.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
48
Hydrogen bonds are attractions
A) between oppositely charged ions.
B) between atoms, resulting in electron sharing.
C) between cations.
D) between atoms, each with partial electrical charges.
E) that rely on hydrophobic interactions.
A) between oppositely charged ions.
B) between atoms, resulting in electron sharing.
C) between cations.
D) between atoms, each with partial electrical charges.
E) that rely on hydrophobic interactions.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
49
Refer to the figure below. 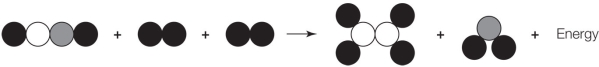 Which statement about the figure is true?
Which statement about the figure is true?
A) It shows a chemical change because the products differ from the reactants.
B) It shows a chemical change because the three molecules were transformed into two molecules.
C) It shows a chemical change because energy was released as a result of the change.
D) It does not accurately show a chemical change because the numbers of atoms on the two sides of the arrow differ.
E) It does not accurately show a chemical change because energy is shown on the wrong side of the arrow.
 Which statement about the figure is true?
Which statement about the figure is true?A) It shows a chemical change because the products differ from the reactants.
B) It shows a chemical change because the three molecules were transformed into two molecules.
C) It shows a chemical change because energy was released as a result of the change.
D) It does not accurately show a chemical change because the numbers of atoms on the two sides of the arrow differ.
E) It does not accurately show a chemical change because energy is shown on the wrong side of the arrow.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
50
A van der Waals interaction is an attraction between
A) the electrons and the nucleus of one molecule.
B) two nonpolar molecules, due to the exclusion of water.
C) the electrons of one molecule and the protons of a nearby molecule.
D) two adjacent nonpolar molecules, due to variations in their electron distribution.
E) two polar molecules, because they are surrounded by water molecules.
A) the electrons and the nucleus of one molecule.
B) two nonpolar molecules, due to the exclusion of water.
C) the electrons of one molecule and the protons of a nearby molecule.
D) two adjacent nonpolar molecules, due to variations in their electron distribution.
E) two polar molecules, because they are surrounded by water molecules.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
51
Some reactions, such as the decomposition of nitroglycerin in dynamite, release large amounts of energy in the form of heat.Others, such as those taking place inside cells, release much smaller amounts of heat.Which statement is true about these reactions?
A) The total amount of energy involved in the cellular reactions is conserved, but new energy is created during the explosive reaction involving nitroglycerin.
B) Though a larger overall change in energy occurs in the nitroglycerin reaction, the total amount of energy present before and after each reaction does not change.
C) Cells use up energy, causing an overall decrease in the total amount of energy present before cellular reactions, while nonliving things, such as dynamite, do not.
D) Both living cells and nonliving things, such as dynamite, cause an overall loss of energy when they release heat during reactions.
E) Only living things conserve energy from their reactions in the form of chemical bond energy, while nonliving things, such as dynamite, lose energy when they react.
A) The total amount of energy involved in the cellular reactions is conserved, but new energy is created during the explosive reaction involving nitroglycerin.
B) Though a larger overall change in energy occurs in the nitroglycerin reaction, the total amount of energy present before and after each reaction does not change.
C) Cells use up energy, causing an overall decrease in the total amount of energy present before cellular reactions, while nonliving things, such as dynamite, do not.
D) Both living cells and nonliving things, such as dynamite, cause an overall loss of energy when they release heat during reactions.
E) Only living things conserve energy from their reactions in the form of chemical bond energy, while nonliving things, such as dynamite, lose energy when they react.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
52
Hydrogen bonds
A) form between two hydrogen atoms.
B) form only between hydrogen and oxygen atoms within a molecule.
C) form only between a weak electronegative atom and hydrogen.
D) involve a transfer of electrons.
E) form weak interactions but can provide structural stability when many are found in a single molecule.
A) form between two hydrogen atoms.
B) form only between hydrogen and oxygen atoms within a molecule.
C) form only between a weak electronegative atom and hydrogen.
D) involve a transfer of electrons.
E) form weak interactions but can provide structural stability when many are found in a single molecule.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
53
When magnesium (Mg) bonds with another element, it
A) gains two electrons from the other element.
B) shares four electrons with the other element.
C) loses two electrons to the other element.
D) forms a hydrogen bond.
E) gains six electrons from the other element.
A) gains two electrons from the other element.
B) shares four electrons with the other element.
C) loses two electrons to the other element.
D) forms a hydrogen bond.
E) gains six electrons from the other element.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
54
Which compound is held together by ionic bonds?
A) Water
B) Sugar
C) Sodium chloride
D) Methane
E) Ammonia
A) Water
B) Sugar
C) Sodium chloride
D) Methane
E) Ammonia

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
55
The ball-and-stick structure of methane (CH4) shows that
A) the molecule is flat.
B) the molecule is not polar.
C) all bonds are hydrogen bonds.
D) all bond angles are different.
E) all bond lengths are different.
A) the molecule is flat.
B) the molecule is not polar.
C) all bonds are hydrogen bonds.
D) all bond angles are different.
E) all bond lengths are different.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
56
Cholesterol is a lipid most often found in cell membranes.It is composed primarily of carbon and hydrogen atoms and has the following chemical formula: C27H46O.Based on this information, one would expect cholesterol to be
A) insoluble in water.
B) a highly polar molecule.
C) a cation.
D) an anion.
E) insoluble in hexane.
A) insoluble in water.
B) a highly polar molecule.
C) a cation.
D) an anion.
E) insoluble in hexane.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
57
Refer to the figure below showing the chemical structures of several molecules. 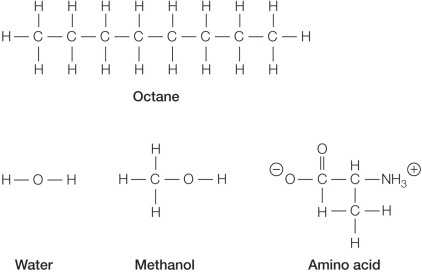 Which pair of molecules is most likely to be miscible (each soluble in the other)?
Which pair of molecules is most likely to be miscible (each soluble in the other)?
A) Octane and water
B) Water and methanol
C) Amino acid and octane
D) Methanol and octane
E) Amino acid and methanol
 Which pair of molecules is most likely to be miscible (each soluble in the other)?
Which pair of molecules is most likely to be miscible (each soluble in the other)?A) Octane and water
B) Water and methanol
C) Amino acid and octane
D) Methanol and octane
E) Amino acid and methanol

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
58
A biologist is conducting experiments on human muscle and collects a variety of data, listed below.Which type of data would provide evidence for the claim that living organisms are chemically dynamic?
A) Amount of force generated by a muscle fiber
B) Length of a muscle fiber
C) Elemental composition of a muscle fiber
D) Rate of metabolism of glucose by a muscle fiber
E) Duration of contraction of a muscle fiber
A) Amount of force generated by a muscle fiber
B) Length of a muscle fiber
C) Elemental composition of a muscle fiber
D) Rate of metabolism of glucose by a muscle fiber
E) Duration of contraction of a muscle fiber

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
59
Particles that have a net negative charge are called
A) electronegative.
B) cations.
C) anions.
D) acids.
E) bases.
A) electronegative.
B) cations.
C) anions.
D) acids.
E) bases.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
60
Refer to the figure showing reactants before chemical change occurs.  Which diagram could represent the products of this change?
Which diagram could represent the products of this change?
A)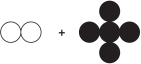
B)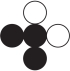
C)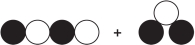
D)
E)
 Which diagram could represent the products of this change?
Which diagram could represent the products of this change?A)

B)

C)
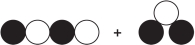
D)

E)


Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
61
Refer to the figures below. 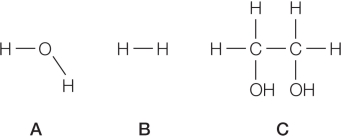 The correct ranking of these compounds in order of lowest to highest heat capacity per mole of compound is B < A < C.Which property would most likely be responsible for this trend?
The correct ranking of these compounds in order of lowest to highest heat capacity per mole of compound is B < A < C.Which property would most likely be responsible for this trend?
A) Molecular weight
B) Number of bonds
C) Hydrogen bonding capacity
D) Molecular shape
E) Ability to dissolve in water
 The correct ranking of these compounds in order of lowest to highest heat capacity per mole of compound is B < A < C.Which property would most likely be responsible for this trend?
The correct ranking of these compounds in order of lowest to highest heat capacity per mole of compound is B < A < C.Which property would most likely be responsible for this trend?A) Molecular weight
B) Number of bonds
C) Hydrogen bonding capacity
D) Molecular shape
E) Ability to dissolve in water

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
62
Surface tension and cohesion occur in pure water because water
A) is nonpolar.
B) contains covalent bonds.
C) forms intermolecular hydrogen bonds.
D) resists changes in temperature.
E) requires high energy input to vaporize.
A) is nonpolar.
B) contains covalent bonds.
C) forms intermolecular hydrogen bonds.
D) resists changes in temperature.
E) requires high energy input to vaporize.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
63
When exposed to extreme heat, the human body relies on _______ to absorb excess calories of heat and maintain normal body temperature.
A) evaporation
B) condensation
C) respiration
D) transpiration
E) degradation
A) evaporation
B) condensation
C) respiration
D) transpiration
E) degradation

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
64
Which compound containing 1H, 12C, and/or 16O has the greatest number of molecules in a sample with a mass of 2 g?
A) Water (H2O)
B) Carbon dioxide (CO2)
C) Acetic acid (CH3OOH)
D) Carbonic acid (H2CO3)
E) Table sugar (C12H22O11)
A) Water (H2O)
B) Carbon dioxide (CO2)
C) Acetic acid (CH3OOH)
D) Carbonic acid (H2CO3)
E) Table sugar (C12H22O11)

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
65
Which characteristic of water contributes most to the relatively constant temperatures of the oceans?
A) Water has the ability to ionize slightly.
B) Water has a high specific heat.
C) Salt water has low surface tension.
D) Salt water is denser than freshwater.
E) Water requires a small amount of heat energy to raise its temperature.
A) Water has the ability to ionize slightly.
B) Water has a high specific heat.
C) Salt water has low surface tension.
D) Salt water is denser than freshwater.
E) Water requires a small amount of heat energy to raise its temperature.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
66
Which statement explains why ice floats in liquid water?
A) As water molecules go from the liquid to the solid state, their rate of motion decreases.
B) Water molecules maintain the same bent shape in liquid and solid states.
C) The ordered lattice structure of water molecules in ice is maintained by hydrogen bonds.
D) The arrangement of water molecules in ice results in fewer molecules per unit volume than in liquid water.
E) Water molecules maintain the same mass as they transition from the liquid to the solid state.
A) As water molecules go from the liquid to the solid state, their rate of motion decreases.
B) Water molecules maintain the same bent shape in liquid and solid states.
C) The ordered lattice structure of water molecules in ice is maintained by hydrogen bonds.
D) The arrangement of water molecules in ice results in fewer molecules per unit volume than in liquid water.
E) Water molecules maintain the same mass as they transition from the liquid to the solid state.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
67
Refer to the balanced chemical equation below.
CH4 + 2 O2 CO2 + 2 H2O + 890 kJ
Which graph represents the energy changes accompanying this reaction?
A)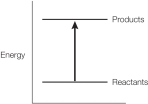
B)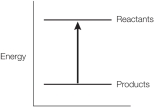
C)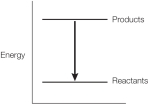
D)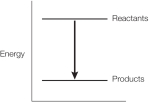
E)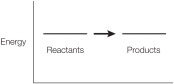
CH4 + 2 O2 CO2 + 2 H2O + 890 kJ
Which graph represents the energy changes accompanying this reaction?
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
68
What features of the water molecule are responsible for its high heat of vaporization, and what other compound shares these features?
A) Water's small size and low molecular weight; carbon dioxide (CO2)
B) Water's polarity and its ability to form intermolecular hydrogen bonds; ammonia (NH3)
C) Water's single bonds and tetrahedral bond orientations; methane (CH4)
D) Water's bent shape and lone pairs of electrons; sulfur dioxide (SO2)
E) Water's covalent bonds involving hydrogen and oxygen atoms; hydrogen peroxide (HOOH)
A) Water's small size and low molecular weight; carbon dioxide (CO2)
B) Water's polarity and its ability to form intermolecular hydrogen bonds; ammonia (NH3)
C) Water's single bonds and tetrahedral bond orientations; methane (CH4)
D) Water's bent shape and lone pairs of electrons; sulfur dioxide (SO2)
E) Water's covalent bonds involving hydrogen and oxygen atoms; hydrogen peroxide (HOOH)

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
69
Refer to the reaction shown. C3H8 + 5 O2 3 CO2 + 4 H2O + energy
Which statement about the reaction is true?
A) O2 is a product.
B) Chemical bonds are conserved during the reaction.
C) The same atoms are present before and after the reaction.
D) A net input of energy is needed for this reaction.
E) The products are similar to the reactants.
Which statement about the reaction is true?
A) O2 is a product.
B) Chemical bonds are conserved during the reaction.
C) The same atoms are present before and after the reaction.
D) A net input of energy is needed for this reaction.
E) The products are similar to the reactants.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
70
Refer to the figures below. 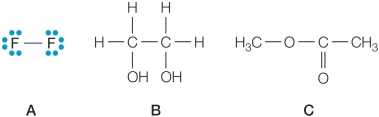 Which compound would have a higher heat of vaporization than water, and why?
Which compound would have a higher heat of vaporization than water, and why?
A) Compound A because it is smaller in size than water.
B) Compound A because unlike water, it is not capable of hydrogen bonding.
C) Compound B because it can form more hydrogen bonds per molecule than water.
D) Compound B because it contains more covalent bonds per molecule than water.
E) Compound C because it contains more oxygen atoms per molecule than water.
 Which compound would have a higher heat of vaporization than water, and why?
Which compound would have a higher heat of vaporization than water, and why?A) Compound A because it is smaller in size than water.
B) Compound A because unlike water, it is not capable of hydrogen bonding.
C) Compound B because it can form more hydrogen bonds per molecule than water.
D) Compound B because it contains more covalent bonds per molecule than water.
E) Compound C because it contains more oxygen atoms per molecule than water.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
71
Phosphate ion has the structure PO43−.This ion is reversibly added to and removed from many different protein molecules in the cell as a means of regulating the proteins' functions.Water's solvent properties are important in understanding the modification of proteins by phosphate ion because
A) biochemists use water as the solvent in laboratory experiments aimed at mimicking the internal workings of a cell.
B) when proteins are removed from cells and placed in solvents from the lab, their functions change.
C) the external cell environment is composed of water, and many signaling molecules move through aqueous environments to bind to receptors on cell surfaces.
D) the internal cell environment is composed of water, and all cellular reactions occur within this aqueous solvent.
E) the phosphate ion has different solubilities in different solvents.
A) biochemists use water as the solvent in laboratory experiments aimed at mimicking the internal workings of a cell.
B) when proteins are removed from cells and placed in solvents from the lab, their functions change.
C) the external cell environment is composed of water, and many signaling molecules move through aqueous environments to bind to receptors on cell surfaces.
D) the internal cell environment is composed of water, and all cellular reactions occur within this aqueous solvent.
E) the phosphate ion has different solubilities in different solvents.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
72
In the summer, ice is used to cool beverages primarily because it
A) floats.
B) is inexpensive.
C) does not affect taste.
D) is composed only of water.
E) absorbs a lot of heat as it melts.
A) floats.
B) is inexpensive.
C) does not affect taste.
D) is composed only of water.
E) absorbs a lot of heat as it melts.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
73
Which observation makes a strong case that the study of water and its properties is relevant to the study of structural biology?
A) Corals are marine animals that live in close association with photosynthetic algae that supply the corals with a source of food.
B) Some Arctic fish produce antifreeze proteins to prevent ice crystals from forming in their cells.
C) Animals that live in caves their entire lives rely on nutrients brought into their habitats by running water or by other organisms.
D) Ice loses mass as water molecules go from the solid state directly to the gas state.
E) Lake ecosystems can be destroyed by chemical fertilizers carried from farmland into lakes in rain runoff.
A) Corals are marine animals that live in close association with photosynthetic algae that supply the corals with a source of food.
B) Some Arctic fish produce antifreeze proteins to prevent ice crystals from forming in their cells.
C) Animals that live in caves their entire lives rely on nutrients brought into their habitats by running water or by other organisms.
D) Ice loses mass as water molecules go from the solid state directly to the gas state.
E) Lake ecosystems can be destroyed by chemical fertilizers carried from farmland into lakes in rain runoff.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
74
A mole of hydrogen and a mole of carbon have
A) different numbers of molecules.
B) fewer hydrogen atoms than carbon atoms.
C) the same number of molecules.
D) the capacity to form one mole of carbohydrate.
E) a different number of molecules than a mole of oxygen.
A) different numbers of molecules.
B) fewer hydrogen atoms than carbon atoms.
C) the same number of molecules.
D) the capacity to form one mole of carbohydrate.
E) a different number of molecules than a mole of oxygen.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
75
How would you make 100 mL of an aqueous solution with a 0.25 M concentration of a compound that has a molecular weight of 200 Da?
A) Add 25 g of the compound to 100 mL of water.
B) Add 20 g of the compound to 100 mL of water.
C) Measure 2.5 g of the compound and add water until the volume equals 100 mL.
D) Measure 2 g of the compound and add water until the volume equals 100 mL.
E) Measure 5 g of the compound and add water until the volume equals 100 mL.
A) Add 25 g of the compound to 100 mL of water.
B) Add 20 g of the compound to 100 mL of water.
C) Measure 2.5 g of the compound and add water until the volume equals 100 mL.
D) Measure 2 g of the compound and add water until the volume equals 100 mL.
E) Measure 5 g of the compound and add water until the volume equals 100 mL.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
76
Vertebrate animals rely on movement of sodium ions in and out of nerve cells to transmit nerve impulses.Which property of water is relevant to this function, and why?
A) Water's strong adhesive properties, because this property explains the attraction between water and other substances
B) Water's changing density with temperature, because this property allows water to move as it heats up
C) Water's polarity, because this property makes it an effective solvent for charged particles
D) Water's high surface tension, because this property allows water to act as a surface that cannot be penetrated easily
E) Water's strong cohesive properties, because this property explains the attraction between water and itself
A) Water's strong adhesive properties, because this property explains the attraction between water and other substances
B) Water's changing density with temperature, because this property allows water to move as it heats up
C) Water's polarity, because this property makes it an effective solvent for charged particles
D) Water's high surface tension, because this property allows water to act as a surface that cannot be penetrated easily
E) Water's strong cohesive properties, because this property explains the attraction between water and itself

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
77
To determine the number of molecules in a teaspoon of sugar, you have to know
A) the mass of the sugar.
B) the mass and density of the sugar.
C) the molecular weight of the sugar.
D) Avogadro's number.
E) the mass and molecular weight of the sugar, and Avogadro's number.
A) the mass of the sugar.
B) the mass and density of the sugar.
C) the molecular weight of the sugar.
D) Avogadro's number.
E) the mass and molecular weight of the sugar, and Avogadro's number.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
78
Ice floats because the ice crystals
A) contain fewer water molecules per volume than the liquid water.
B) are more dense than liquid water.
C) form heat, which makes water expand.
D) can move quickly and therefore can float.
E) have a high surface tension.
A) contain fewer water molecules per volume than the liquid water.
B) are more dense than liquid water.
C) form heat, which makes water expand.
D) can move quickly and therefore can float.
E) have a high surface tension.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
79
Some frog species lay their eggs in shallow waters.After fertilization, the embryos develop into tadpoles that require an aquatic environment until they develop into adults.This can be challenging in shallow waters, especially in regions where the daytime temperatures can reach the upper 90s in Fahrenheit degrees.Which two properties of water are most responsible for improving the odds of tadpole survival in these shallow waters on hot days?
A) High surface tension and strong adhesive forces
B) Strong cohesive and adhesive forces
C) High heat capacity and high surface tension
D) High heat of vaporization and strong adhesive forces
E) High heat capacity and high heat of vaporization
A) High surface tension and strong adhesive forces
B) Strong cohesive and adhesive forces
C) High heat capacity and high surface tension
D) High heat of vaporization and strong adhesive forces
E) High heat capacity and high heat of vaporization

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck
80
A car sitting in the sun on a hot summer day becomes very hot to the touch.Water in a bucket sitting next to the car under the same conditions for the same length of time feels cool to the touch.Which statement explains this difference?
A) Radiant energy goes into breaking the forces of attraction between water molecules before increasing their rate of motion.
B) Radiant energy is reflected off the surface of water rather than being absorbed by the water molecules.
C) Radiant energy cannot easily penetrate water because of its density and is therefore not absorbed readily.
D) Radiant energy is absorbed poorly by liquids, compared with solids.
E) Radiant energy is absorbed by certain elements more readily than by other elements.
A) Radiant energy goes into breaking the forces of attraction between water molecules before increasing their rate of motion.
B) Radiant energy is reflected off the surface of water rather than being absorbed by the water molecules.
C) Radiant energy cannot easily penetrate water because of its density and is therefore not absorbed readily.
D) Radiant energy is absorbed poorly by liquids, compared with solids.
E) Radiant energy is absorbed by certain elements more readily than by other elements.

Unlock Deck
Unlock for access to all 246 flashcards in this deck.
Unlock Deck
k this deck



