Deck 5: Chemical Bonds Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 5: Chemical Bonds Compounds
1
What is the CORRECT Lewis symbol for a sulfur atom?
A)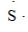
B)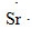
C)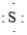
D)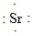
A)
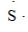
B)
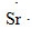
C)
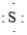
D)
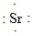
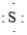
2
Which formula is the CORRECT one for a sulfite ion?
A) S2-
B) SH4+
C) SO42-
D) SO32-
A) S2-
B) SH4+
C) SO42-
D) SO32-
SO32-
3
The electronic structure of the aluminum atom is [Ne]3s23p1.The electronic structure of the aluminum ion is:
A) [Ne]3s23p4.
B) [Ne]3s2.
C) [Ne]3s1.
D) [Ne].
A) [Ne]3s23p4.
B) [Ne]3s2.
C) [Ne]3s1.
D) [Ne].
[Ne].
4
How many valence electrons does a neutral oxygen atom have?
A) two
B) six
C) seven
D) eight
A) two
B) six
C) seven
D) eight

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
Members of the alkaline earth metals form ions with a charge of:
A) +1.
B) +2.
C) -1.
D) -2.
A) +1.
B) +2.
C) -1.
D) -2.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
The empirical formula for iron(II)nitrate is:
A) Fe2NO3.
B) Fe(NO3)2.
C) Fe(NO2)2.
D) FeNO3.
A) Fe2NO3.
B) Fe(NO3)2.
C) Fe(NO2)2.
D) FeNO3.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
Which formula is the CORRECT one for a phosphate ion?
A) P3-
B) PO43-
C) PH4+
D) PO32-
A) P3-
B) PO43-
C) PH4+
D) PO32-

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
Members of the halogen family typically form ions with a charge of:
A) +1.
B) +2.
C) -1.
D) -2.
A) +1.
B) +2.
C) -1.
D) -2.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
What is the CORRECT Lewis symbol for a carbon atom?
A)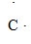
B)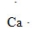
C)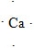
D)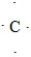
A)
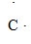
B)
C)
D)
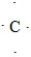

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
The empirical formula for aluminum oxide is:
A) AlO.
B) AlO3.
C) Al3O2.
D) Al2O3.
A) AlO.
B) AlO3.
C) Al3O2.
D) Al2O3.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
What is the charge on a sulfide ion?
A) +1
B) +2
C) -1
D) -2
A) +1
B) +2
C) -1
D) -2

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
How many valence electrons does a neutral nitrogen atom have?
A) three
B) five
C) seven
D) eight
A) three
B) five
C) seven
D) eight

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
The empirical formula for copper(I)nitrite is:
A) Co2NO2.
B) Co(NO2)2.
C) Cu(NO2)2.
D) CuNO2.
A) Co2NO2.
B) Co(NO2)2.
C) Cu(NO2)2.
D) CuNO2.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
How many valence electrons does a neutral fluorine atom have?
A) one
B) seven
C) eight
D) nine
A) one
B) seven
C) eight
D) nine

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
The electronic structure of the nitrogen atom is [He]2s22p3.The electronic structure of the nitride ion is:
A) [Ar].
B) [He]2s2.
C) [He]2s22p2.
D) [He]2s22p1.
A) [Ar].
B) [He]2s2.
C) [He]2s22p2.
D) [He]2s22p1.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
The empirical formula for magnesium nitride :
A) MgN.
B) MgN3.
C) Mg3N2.
D) Mg2N3.
A) MgN.
B) MgN3.
C) Mg3N2.
D) Mg2N3.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
What is the CORRECT Lewis symbol for a phosphorus atom?
A)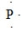
B)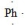
C)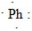
D)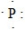
A)
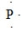
B)
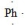
C)
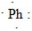
D)
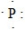

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
What is the charge on an oxide ion?
A) +1
B) +2
C) -1
D) -2
A) +1
B) +2
C) -1
D) -2

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
The empirical formula for copper(II)nitrite is:
A) Co2NO2.
B) Co(NO2)2.
C) Cu(NO2)2.
D) CuNO2.
A) Co2NO2.
B) Co(NO2)2.
C) Cu(NO2)2.
D) CuNO2.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
Which formula is the CORRECT one for a sulfate ion?
A) S2-
B) SH4+
C) SO42-
D) SO32-
A) S2-
B) SH4+
C) SO42-
D) SO32-

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
When two nonmetals combine,they "share" the electrons between them.This type of bond is a(n)_____ bond.
A) covalent
B) ionic
C) metallic
D) municipal
A) covalent
B) ionic
C) metallic
D) municipal

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
The molecular formula for this compound is: 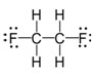
A) CH2FC
B) C2H4F2
C) C2(HF)3
D) CF2(H2)

A) CH2FC
B) C2H4F2
C) C2(HF)3
D) CF2(H2)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
The systematic name of the compound N2O4 is:
A) dinitrogen tetroxide.
B) dinitrogen oxide.
C) nitrogen oxide.
D) mononitrogen dioxide.
A) dinitrogen tetroxide.
B) dinitrogen oxide.
C) nitrogen oxide.
D) mononitrogen dioxide.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
The molecular formula for this compound is: 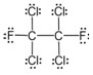
A) CFCl2
B) C2F2Cl4
C) C2(FCl)3
D) CF2(Cl2)

A) CFCl2
B) C2F2Cl4
C) C2(FCl)3
D) CF2(Cl2)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
Which compound is covalent?
A) CBr4
B) PbCl2
C) NH4NO3
D) MgF2
A) CBr4
B) PbCl2
C) NH4NO3
D) MgF2

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
Which compound is covalent?
A) CO2
B) FeBr2
C) NH4NO3
D) MgCl2
A) CO2
B) FeBr2
C) NH4NO3
D) MgCl2

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
The systematic name of the compound S2Cl2 is:
A) sulfur chloride.
B) disulfur dichlorine.
C) disulfur dichloride.
D) sulfur dichloride.
A) sulfur chloride.
B) disulfur dichlorine.
C) disulfur dichloride.
D) sulfur dichloride.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
When a metal and a nonmetal combine,they "transfer" the electrons between them.This type of bond is a(n)_____ bond.
A) covalent
B) ionic
C) metallic
D) municipal
A) covalent
B) ionic
C) metallic
D) municipal

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
The systematic name for the compound NCl3 is:
A) trichloronitride.
B) chlorine nitride.
C) mononitrogen trichloride.
D) nitrogen trichloride.
A) trichloronitride.
B) chlorine nitride.
C) mononitrogen trichloride.
D) nitrogen trichloride.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
Which compound is ionic?
A) CS2
B) NO3
C) SO2
D) MgCl2
A) CS2
B) NO3
C) SO2
D) MgCl2

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
What is the empirical formula for hydrazine,N2H4?
A) N2H4
B) NH2
C) NH
D) 2NH
A) N2H4
B) NH2
C) NH
D) 2NH

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
The systematic name for the compound PF5 is:
A) pentafluorophosphate.
B) phosphorus pentafluoride.
C) fluorine phosphide.
D) monophosphorus pentafluorate.
A) pentafluorophosphate.
B) phosphorus pentafluoride.
C) fluorine phosphide.
D) monophosphorus pentafluorate.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
The systematic name for the compound SO3 is:
A) sulfur trioxide.
B) monosulfur trioxide.
C) sulfur oxide.
D) monosulfur dioxide.
A) sulfur trioxide.
B) monosulfur trioxide.
C) sulfur oxide.
D) monosulfur dioxide.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
The empirical formula for zinc sulfide is:
A) ZnS.
B) ZnS2.
C) Zn3S2.
D) Zn2S2.
A) ZnS.
B) ZnS2.
C) Zn3S2.
D) Zn2S2.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
What is the empirical formula for benzene,C6H6?
A) C6H6
B) C3H3
C) C2H2
D) CH
A) C6H6
B) C3H3
C) C2H2
D) CH

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
The systematic name for the compound K3PO4 is:
A) potassium phosphorus oxide.
B) potassium phosphide.
C) potassium phosphate.
D) tripotassium monophosphite.
A) potassium phosphorus oxide.
B) potassium phosphide.
C) potassium phosphate.
D) tripotassium monophosphite.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
What is the empirical formula for glucose,C6H12O6?
A) C6H12O6
B) CH2O
C) C2H4O2
D) CHO
A) C6H12O6
B) CH2O
C) C2H4O2
D) CHO

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
The systematic name for the compound Co2(SO3)3 is:
A) cobalt sulfite.
B) cobalt(III)sulfite.
C) cobalt(II)sulfite.
D) cobalt sulfide.
A) cobalt sulfite.
B) cobalt(III)sulfite.
C) cobalt(II)sulfite.
D) cobalt sulfide.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
The systematic name for the compound Fe2(SO4)3 is:
A) iron sulfate.
B) iron(III)sulfate.
C) iron(II)sulfate.
D) iron sulfur oxide.
A) iron sulfate.
B) iron(III)sulfate.
C) iron(II)sulfate.
D) iron sulfur oxide.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
The systematic name for the compound K2SO4 is:
A) potassium sulfate.
B) potassium sulfide.
C) potassium sulfur oxide.
D) dipotassium monosulfide.
A) potassium sulfate.
B) potassium sulfide.
C) potassium sulfur oxide.
D) dipotassium monosulfide.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
Covalent compounds that form H+ ions in solution are called:
A) colloids.
B) bases.
C) isotopes.
D) acids.
A) colloids.
B) bases.
C) isotopes.
D) acids.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
What is the CORRECT formula for hydrochloric acid?
A) HCl
B) HClO
C) HClO2
D) HClO3
A) HCl
B) HClO
C) HClO2
D) HClO3

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
The compound SF6 is composed of:
A) polyatomic ions.
B) polycarbonate blends.
C) an ionic lattice.
D) discrete molecules.
A) polyatomic ions.
B) polycarbonate blends.
C) an ionic lattice.
D) discrete molecules.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
The compound CO2 is composed of:
A) polyatomic ions.
B) polycarbonate blends.
C) an ionic lattice.
D) discrete molecules.
A) polyatomic ions.
B) polycarbonate blends.
C) an ionic lattice.
D) discrete molecules.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
What is the name of the binary acid,HI?
A) hydroiodic acid
B) iodic acid
C) iodous acid
D) hydroiodous acid
A) hydroiodic acid
B) iodic acid
C) iodous acid
D) hydroiodous acid

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
What is the CORRECT formula for hydrosulfuric acid?
A) H2S
B) H2SO3
C) H2SO4
D) HS
A) H2S
B) H2SO3
C) H2SO4
D) HS

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
What is the name of the binary acid,HBr?
A) hydrobromic acid
B) bromic acid
C) bromous acid
D) hydrobromous acid
A) hydrobromic acid
B) bromic acid
C) bromous acid
D) hydrobromous acid

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
The compound NaCl is composed of:
A) polyatomic ions.
B) polycarbonate blends.
C) an ionic lattice.
D) discrete molecules.
A) polyatomic ions.
B) polycarbonate blends.
C) an ionic lattice.
D) discrete molecules.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
What is the CORRECT formula for phosphoric acid?
A) H3P
B) H3PO2
C) H3PO3
D) H3PO4
A) H3P
B) H3PO2
C) H3PO3
D) H3PO4

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
Which compound,when dissolved in water,will NOT result in dissociation?
A) table salt,NaCl
B) table sugar,C12H22O11
C) baking soda,NaHCO3
D) Epsom salts,MgSO4
A) table salt,NaCl
B) table sugar,C12H22O11
C) baking soda,NaHCO3
D) Epsom salts,MgSO4

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
The compound MgF2 is composed of:
A) polyatomic ions.
B) polycarbonate blends.
C) an ionic lattice.
D) discrete molecules.
A) polyatomic ions.
B) polycarbonate blends.
C) an ionic lattice.
D) discrete molecules.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
What is the name of the oxyacid,H2SO4?
A) hydrosulfuric acid
B) sulfuric acid
C) sulfurous acid
D) hydrosulfurous acid
A) hydrosulfuric acid
B) sulfuric acid
C) sulfurous acid
D) hydrosulfurous acid

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
What is the name of the oxyacid,H2CO3?
A) hydrocarbonic acid
B) carbonic acid
C) carbonous acid
D) hydrocarbonous acid
A) hydrocarbonic acid
B) carbonic acid
C) carbonous acid
D) hydrocarbonous acid

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
Which compound is ionic?
A) SO3
B) NF3
C) NH3
D) NaCl
A) SO3
B) NF3
C) NH3
D) NaCl

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
What is the CORRECT formula for nitric acid?
A) HNO2
B) HN
C) HNO3
D) HNO4
A) HNO2
B) HN
C) HNO3
D) HNO4

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
Which compound,when dissolved in water,will result in dissociation?
A) table salt,NaCl
B) methyl alcohol,CH4O
C) antifreeze,C2H6O2
D) acetone,C3H6O
A) table salt,NaCl
B) methyl alcohol,CH4O
C) antifreeze,C2H6O2
D) acetone,C3H6O

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck



