Deck 16: Ð Bonds With Hidden Leaving Groups
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/81
Play
Full screen (f)
Deck 16: Ð Bonds With Hidden Leaving Groups
1
What pH range best supports imine formation?
A)0-2
B)2-4
C)4-6
D)6-8
A)0-2
B)2-4
C)4-6
D)6-8
4-6
2
What is the product of the reaction shown below? 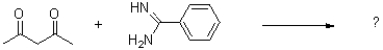
A)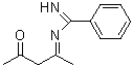
B)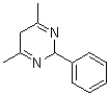
C)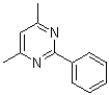
D)No reaction would occur.

A)

B)

C)

D)No reaction would occur.

3
What is the product (or products)of the following reaction? 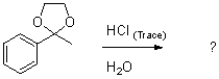
A)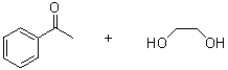
B)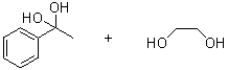
C)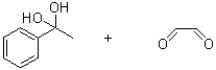
D)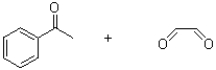

A)
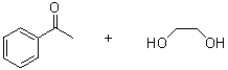
B)
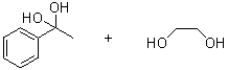
C)
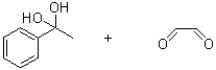
D)
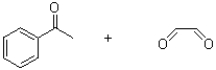
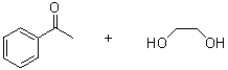
4
What is the functional group shown below? 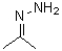
A)imine
B)oxime
C)hydrazine
D)hydrazone

A)imine
B)oxime
C)hydrazine
D)hydrazone

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
5
What is the functional group shown below? 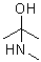
A)imine
B)amine
C)aminal
D)hemiaminal

A)imine
B)amine
C)aminal
D)hemiaminal

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
6
What is the common name for the following heterocycle? 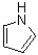
A)furan
B)thiofuran
C)pyridine
D)pyrrole

A)furan
B)thiofuran
C)pyridine
D)pyrrole

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
7
What conditions and reagents will best accomplish the following transformation? 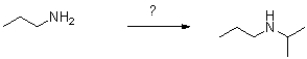
A)1)Propanone,H3O+ 2)NaCNBH3,3)H3O+
B)2-Bromoproane,Et3N,DMF
C)2-Propanol,H3O+
D)Ethanoic acid,DCC

A)1)Propanone,H3O+ 2)NaCNBH3,3)H3O+
B)2-Bromoproane,Et3N,DMF
C)2-Propanol,H3O+
D)Ethanoic acid,DCC

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
8
What is the product of the transformation shown below? 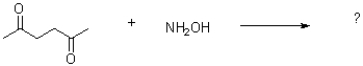
A)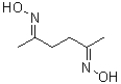
B)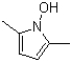
C)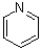
D)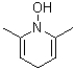

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following best describes the carbohydrate shown below? 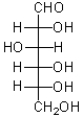
A)á
B)â
C)D
D)L

A)á
B)â
C)D
D)L

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
10
What reagents and conditions are required for the Wolff-Kischner reduction?
A)1)NH2NH2,2)KOH,heat
B)1)MgBrCH3,2)H3O+
C)MeNH2,NaCNBH3,H3O+
D)P2O5
A)1)NH2NH2,2)KOH,heat
B)1)MgBrCH3,2)H3O+
C)MeNH2,NaCNBH3,H3O+
D)P2O5

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
11
Figure 1 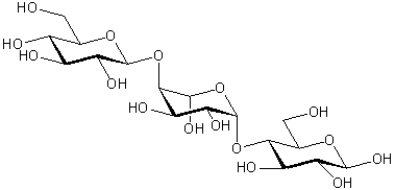
Referring to Figure 1,how many acetal functional groups are present in the trisaccharide?
A)0
B)1
C)2
D)3

Referring to Figure 1,how many acetal functional groups are present in the trisaccharide?
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
12
What is the product of the transformation shown below? 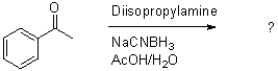
A)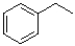
B)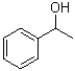
C)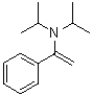
D)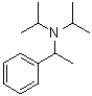
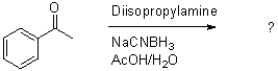
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
13
Figure 1 
Referring to Figure 1,from left to right,what is the configuration of each of the three anomeric carbons?
A)â,â,â
B)á,â,á
C)â,á,â
D)á á á

Referring to Figure 1,from left to right,what is the configuration of each of the three anomeric carbons?
A)â,â,â
B)á,â,á
C)â,á,â
D)á á á

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
14
Which conditions are required for the following transformation? 
A)HCl,H2O
B)KOH,heat
C)NaCNBH3
D)Pd,H2

A)HCl,H2O
B)KOH,heat
C)NaCNBH3
D)Pd,H2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
15
What is the functional group shown below? 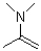
A)imine
B)amine
C)enamine
D)oxime

A)imine
B)amine
C)enamine
D)oxime

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
16
Figure 1 
Referring to Figure 1,how many hemiacetal functional groups are present in the trisaccharide?
A)0
B)1
C)2
D)3

Referring to Figure 1,how many hemiacetal functional groups are present in the trisaccharide?
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following products will result from the heterocyclic condensation reaction shown below? 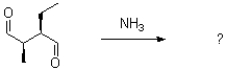
A)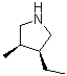
B)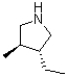
C)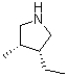
D)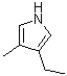

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
18
What is the functional group shown below? 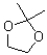
A)ether
B)acetal
C)hemiacetal
D)oxonium

A)ether
B)acetal
C)hemiacetal
D)oxonium

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
19
What is the role of P2O5 in the reaction shown below? 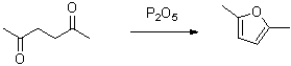
A)to activate the carbonyl as an electrophile
B)to deprotonate the ketone
C)to bring the oxygens together for cyclization
D)to absorb the resultant water formed in the reaction

A)to activate the carbonyl as an electrophile
B)to deprotonate the ketone
C)to bring the oxygens together for cyclization
D)to absorb the resultant water formed in the reaction

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
20
What is the intermediate of the transformation shown below? 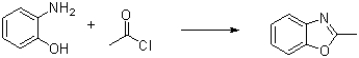
A)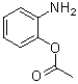
B)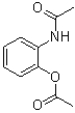
C)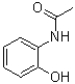
D)The reaction is concerted and no intermediate is formed.
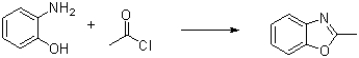
A)

B)

C)

D)The reaction is concerted and no intermediate is formed.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following imines will NOT form an imine with a ketone?
A)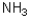
B)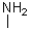
C)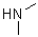
D)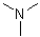
A)
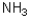
B)
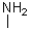
C)
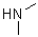
D)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
22
What is the major product of the reaction scheme shown below? 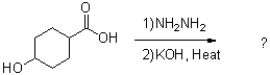
A)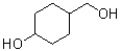
B)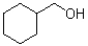
C)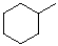
D)No reaction would occur.

A)

B)

C)

D)No reaction would occur.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
23
What is the role of sulfuric acid in the following reaction? 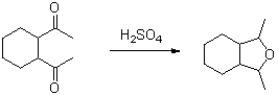
A)nucleophile
B)catalyst
C)solvent
D)dehydrating agent

A)nucleophile
B)catalyst
C)solvent
D)dehydrating agent

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is NOT a possible reaction scheme to obtain the final product shown below? 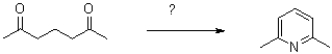
A)1)NH3 2)HNO3/H2SO4
B)NH2OH,H3O+
C)1)NH3 2)P2O5
D)1)NH3 2)DDQ
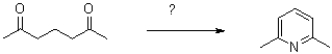
A)1)NH3 2)HNO3/H2SO4
B)NH2OH,H3O+
C)1)NH3 2)P2O5
D)1)NH3 2)DDQ

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following structures represents a hemiaminal?
A)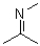
B)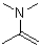
C)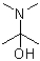
D)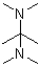
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following amines will most readily form an enamine with a ketone?
A)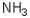
B)
C)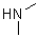
D)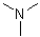
A)
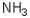
B)
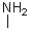
C)
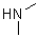
D)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
27
An amine and a ketone can condense to form an imine.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
28
What is the major product of the reaction scheme shown below? 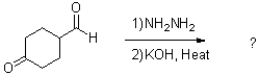
A)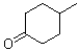
B)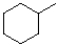
C)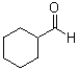
D)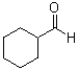

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
29
What is the major product of the reaction scheme shown below? 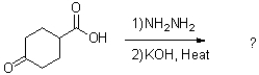
A)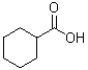
B)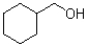
C)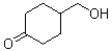
D)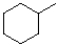

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
30
Which conditions would push the forward direction (I)and the reverse direction (II)in the following reaction scheme? 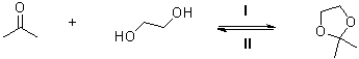
A)(I)- H+ (II)- H+
B)(I)- H+ (II)- H+ /H2O
C)(I)- H+ /H2O (II)- H+
D)(I)- H+ /H2O II)- H+ /H2O

A)(I)- H+ (II)- H+
B)(I)- H+ (II)- H+ /H2O
C)(I)- H+ /H2O (II)- H+
D)(I)- H+ /H2O II)- H+ /H2O

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
31
What reagents would accomplish the following transformation? 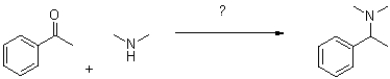
A)KOH,heat
B)AcOH,H2O
C)NaCNBH3,AcOH,H2O
D)DDQ,H2O
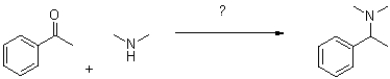
A)KOH,heat
B)AcOH,H2O
C)NaCNBH3,AcOH,H2O
D)DDQ,H2O

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
32
What would be the major product of the following reaction? 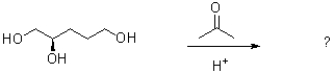
A)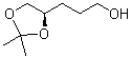
B)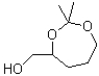
C)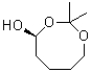
D)No reaction would occur.
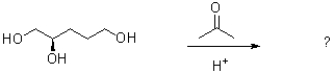
A)

B)

C)

D)No reaction would occur.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
33
The products of acetal formation from a ketone are shown below.Which reactant did the oxygen in water come from? 
A)acetone
B)methanol
C)50% acetone,50% methanol
D)TsOH

A)acetone
B)methanol
C)50% acetone,50% methanol
D)TsOH

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
34
What is the major product of the reaction shown below? 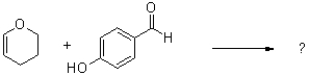
A)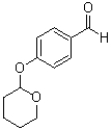
B)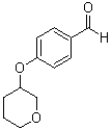
C)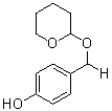
D)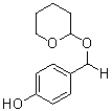

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
35
What would be the product of the following reaction? 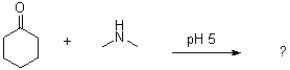
A)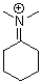
B)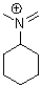
C)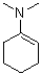
D)No reaction would occur.
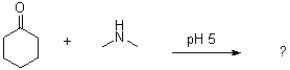
A)

B)

C)

D)No reaction would occur.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
36
Which reagents are required to achieve the following transformation? 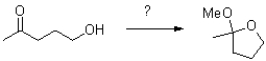
A)HCl(trace), H2O
B)HCl(trace), MeOH
C)HCl(trace), H2O,MeOH
D)HCl(trace),
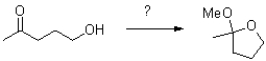
A)HCl(trace), H2O
B)HCl(trace), MeOH
C)HCl(trace), H2O,MeOH
D)HCl(trace),

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
37
Acetal formation is reversible.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
38
When the hydroxyl group of a cyclic carbohydrate at the anomeric position is axial,it is designated á.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
39
Hydrazine and ketone can condense to form a semicarbazone.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
40
What would be the major product of the following reaction? 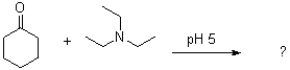
A)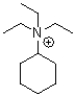
B)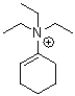
C)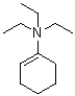
D)No reaction would occur.

A)

B)

C)

D)No reaction would occur.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
41
The anomeric carbon is the penultimate carbon of a linear carbohydrate.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
42
Water is the hidden leaving group of carbonyls.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
43
The carbonyl oxygen in a ketone/aldehyde is retained when transformed into an acetal.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
44
The first mechanistic step in forming an acetal from an aldehyde is nucleophilic attack on the carbonyl by an alcohol.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
45
DHF is used as a protecting group for aldehydes and ketones.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
46
Anomers are the result of the formation of an sp3 carbon through the hemiacetal formation of cyclic sugars.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
47
Cyclic acetals form faster than open chain acetals.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
48
A pyrrole is a sulfur containing heterocycle.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
49
A Dean-Stark trap is used to drive the acetal formation reaction forward.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
50
Pyridine can be produced from hydroxylamine and a 1,5-dione

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
51
Cyclic hemiacetals are more common than open-chain acetals.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
52
Acetals can protect aldehydes and ketones from Grignard reactions.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
53
1,2 diols form acetals faster than alcohols with ketones and aldehydes.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
54
P2O5 is used in the production of furans.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
55
Enamines are most readily formed with secondary amines.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
56
Aldehydes form acetals faster than ketones.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
57
Acetal formation is both acid and base catalyzed.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
58
The D and L designations of carbohydrates represent the orientation of the penultimate carbon in the fisher projection.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
59
A quantitative amount of acid is required to convert an acetal back to an aldehyde and diol.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
60
Dihydropyran is used as a protecting group for aldehydes.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
61
_______________ is the common name for an aromatic five-membered ring structure containing an endocyclic nitrogen atom.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
62
A â sugar is one where the anomeric hydroxyl group and the furthest ring substituent are _______________ to each other.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
63
Propose a mechanism for the reaction shown below. 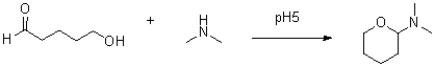


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
64
Draw a mechanism for the following reaction. 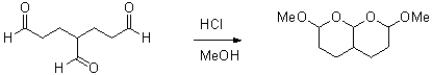


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
65
Hydroxylamine and ketones condense with each other to form a(n)_______________ .

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
66
Identify all the products formed when the compound below is treated with aqueous acid. 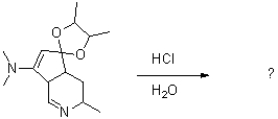


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
67
A change to a functional group to reduce its chemical reactivity is referred to as a(n)_______________ .

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
68
A D sugar is one where the hydroxyl group on the _______________ carbon points to the right in the Ficsher projection.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
69
Cyclization of a carbohydrate leads to the formation of an acetal.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
70
Propose a mechanism for the transformation shown below. 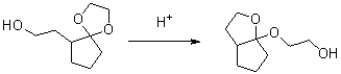


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
71
The reaction of an aldehyde and an amine forms a(n)_______________ .

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
72
The condensation of an amine with a ketone or aldehyde to form an imine occurs fastest around pH 5.The rate of reaction slows as the pH increases and decreases.Explain this observation.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
73
Propose a mechanism for the following transformation. 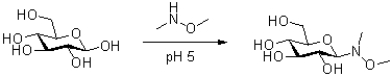


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
74
Show the mechanism of the following reaction. 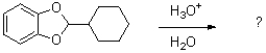
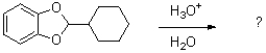

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
75
An aromatic 5-membered ring containing an oxygen atom is known as a(n)_______________ .

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
76
Suggest a sequence of reactions that would accomplish the following transformation.Show all intermediate molecules made. 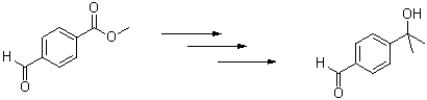


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
77
Propose a synthetic scheme to obtain the following product.Show all intermediate structures. 


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
78
_______________ is required to make furan from a di-one.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
79
The condensation of hydrazine with a ketone forms a(n)_______________ .

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
80
DDQ is used as a(n)_______________ in the formation of pyridine derivatives.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck



