Deck 9: Conjugation and Aromaticity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/80
Play
Full screen (f)
Deck 9: Conjugation and Aromaticity
1
How many atoms are part of the conjugated system of the molecule shown below? 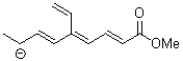
A)5
B)8
C)11
D)12

A)5
B)8
C)11
D)12
12
2
Which of the following is considered non-aromatic?
A)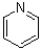
B)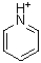
C)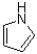
D)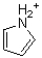
A)

B)

C)
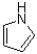
D)
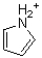
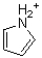
3
Which of the following does NOT represent a conjugated system?
A)
B)
C)
D)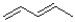
A)

B)

C)

D)
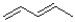
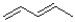
4
What is the configuration around the centre sigma bond of butadiene shown below? 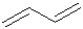
A)cis
B)trans
C)s-cis
D)s-trans

A)cis
B)trans
C)s-cis
D)s-trans

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
5
Which resonance form of anthracene contributes more towards it's stability? 
A)A because it's more aromatic
B)A because it's less aromatic
C)B because it's more aromatic
D)B because it's less aromatic

A)A because it's more aromatic
B)A because it's less aromatic
C)B because it's more aromatic
D)B because it's less aromatic

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following best represents the HOMO of butadiene shown below? 
A)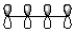
B)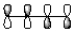
C)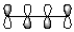
D)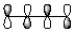

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following best represents the HOMO of the pentadienyl anion shown below? 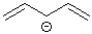
A)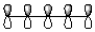
B)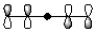
C)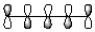
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following best represents the LUMO of hexatriene shown below? 
A)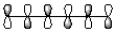
B)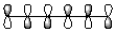
C)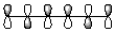
D)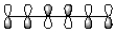

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
9
How many nitrogen lone pairs are involved in the aromaticity of the following molecule? 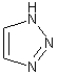
A)0
B)1
C)2
D)3

A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
10
How many atoms are part of the conjugated system of the molecule shown below? 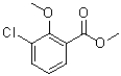
A)6
B)7
C)11
D)13

A)6
B)7
C)11
D)13

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds is considered non-aromatic?
A)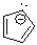
B)
C)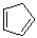
D)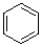
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is NOT a criterion for aromaticity?
A)be cyclic and planar
B)have a p-orbital on all participating ring atoms
C)have 4n+2 number of delocalized ð electrons
D)have an even number of atoms involved in the aromatic system
A)be cyclic and planar
B)have a p-orbital on all participating ring atoms
C)have 4n+2 number of delocalized ð electrons
D)have an even number of atoms involved in the aromatic system

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following best describes the bond length of the central sigma bond in 1,3-butadiene shown below? 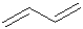
A)It is longer than an average C-C sigma bond.
B)It is shorter than an average C-C sigma bond.
C)It is the same length as an average C-C sigma bond.
D)There is not enough information to determine bond length.

A)It is longer than an average C-C sigma bond.
B)It is shorter than an average C-C sigma bond.
C)It is the same length as an average C-C sigma bond.
D)There is not enough information to determine bond length.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following best describes the molecule shown below? 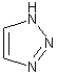
A)aromatic
B)non-aromatic
C)anti-aromatic
D)symmetrical

A)aromatic
B)non-aromatic
C)anti-aromatic
D)symmetrical

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
15
Why does an increase in conjugation lead to an increase in absorption wavelength?
A)Increased conjugation leads to a smaller gap in energy between the HOMO and LUMO.
B)Increased conjugation leads to a larger gap in energy between the HOMO and LUMO.
C)Increased conjugation leads to a smaller gap in energy between the ó and ó* orbitals.
D)Increased conjugation leads to a larger gap in energy between the ó and ó* orbitals.
A)Increased conjugation leads to a smaller gap in energy between the HOMO and LUMO.
B)Increased conjugation leads to a larger gap in energy between the HOMO and LUMO.
C)Increased conjugation leads to a smaller gap in energy between the ó and ó* orbitals.
D)Increased conjugation leads to a larger gap in energy between the ó and ó* orbitals.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
16
Which molecule would be expected to have the higher absorption wavelength? 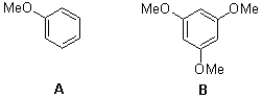
A)A because it's more conjugated
B)A because it's less conjugated
C)B because it's more conjugated
D)B because it's less conjugated

A)A because it's more conjugated
B)A because it's less conjugated
C)B because it's more conjugated
D)B because it's less conjugated

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
17
Compared to ethene,butadiene would be expected to absorb light with which of the following properties?
A)higher wavelength and higher energy
B)higher wavelength and lower energy
C)lower wavelength and higher energy
D)lower wavelength and lower energy
A)higher wavelength and higher energy
B)higher wavelength and lower energy
C)lower wavelength and higher energy
D)lower wavelength and lower energy

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following ions is considered anti-aromatic?
A)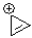
B)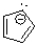
C)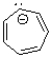
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following best represents the LUMO of the propenyl cation shown below? 
A)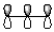
B)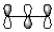
C)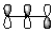
D)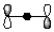

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
20
How many atoms are part of the conjugated system of (+)- Frondonsin B shown below? 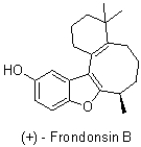
A)5
B)8
C)10
D)12

A)5
B)8
C)10
D)12

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
21
What is the hybridization of the nitrogen atom in the structure shown below? 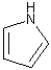
A)s
B)p
C)sp2
D)sp3

A)s
B)p
C)sp2
D)sp3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
22
What is the hybridization of the oxygen atom in furan? 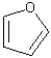
A)sp
B)sp2
C)sp3
D)p

A)sp
B)sp2
C)sp3
D)p

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following best describes the following molecule? 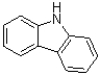
A)aromatic
B)anti-aromatic
C)non-aromatic
D)somewhat aromatic

A)aromatic
B)anti-aromatic
C)non-aromatic
D)somewhat aromatic

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
24
Conjugated systems are more stable than non-conjugated systems.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following best describes double bonds in aromatic systems?
A)They are more susceptible to hydrogenation than non-aromatic double bonds.
B)They are less susceptible to hydrogenation than double bonds.
C)They are equally susceptible to hydrogenation when compared to non-aromatic double bonds.
D)There is no difference between an aromatic and non-aromatic double bond.
A)They are more susceptible to hydrogenation than non-aromatic double bonds.
B)They are less susceptible to hydrogenation than double bonds.
C)They are equally susceptible to hydrogenation when compared to non-aromatic double bonds.
D)There is no difference between an aromatic and non-aromatic double bond.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
26
How many nodal planes does the following representation of benzene have? 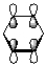
A)0
B)1
C)2
D)3
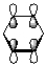
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
27
How many nodal planes does the following representation of benzene have? 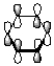
A)1
B)2
C)3
D)6

A)1
B)2
C)3
D)6

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following compounds would liberate the most heat when hydrogenated and why? 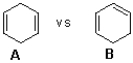
A)A because it's conjugated.
B)A because it's not conjugated.
C)B because it's conjugated.
D)B because it's not conjugated.

A)A because it's conjugated.
B)A because it's not conjugated.
C)B because it's conjugated.
D)B because it's not conjugated.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
29
How many nodal planes does the following representation of benzene have? 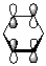
A)0
B)1
C)2
D)3
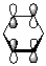
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is an accurate description of benzene?
A)There are two distinct structures in equilibrium.
B)All the carbon-carbon bonds are the same length.
C)Some carbon-carbon bonds are longer than others.
D)There are distinct single and double bonds.
A)There are two distinct structures in equilibrium.
B)All the carbon-carbon bonds are the same length.
C)Some carbon-carbon bonds are longer than others.
D)There are distinct single and double bonds.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
31
s-cis is a more stable confirmation then s-trans.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements best describes the molecule below,assuming planarity? 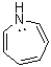
A)It contains 6 ð electrons and is aromatic.
B)It contains 8 ð electrons and is non-aromatic.
C)It contains 8 ð electrons and is aromatic.
D)It contains 8 ð electrons and is anti-aromatic.

A)It contains 6 ð electrons and is aromatic.
B)It contains 8 ð electrons and is non-aromatic.
C)It contains 8 ð electrons and is aromatic.
D)It contains 8 ð electrons and is anti-aromatic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
33
How many ð electron pairs are in the following molecule of benzamide? 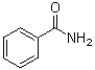
A)3
B)4
C)5
D)6

A)3
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
34
Increased conjugation leads to an increase in absorption wavelength.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
35
In the aromatic compound resonance form shown below,which heteroatoms are contributing lone pairs towards aromaticity? 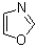
A)nitrogen only
B)oxygen only
C)both nitrogen and oxygen
D)neither nitrogen or oxygen

A)nitrogen only
B)oxygen only
C)both nitrogen and oxygen
D)neither nitrogen or oxygen

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
36
How many electrons pairs are part of the conjugated system in the molecule shown below? 
A)0
B)2
C)3
D)4

A)0
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following best describes anti-aromatic compounds?
A)They are not planar.
B)They are not cyclic.
C)They don't have p orbitals on all participating ring atoms.
D)They don't follow the 4n+2 rule.
A)They are not planar.
B)They are not cyclic.
C)They don't have p orbitals on all participating ring atoms.
D)They don't follow the 4n+2 rule.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
38
How many ð electron pairs are present in the following aromatic heterocycle? 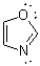
A)2
B)3
C)4
D)5
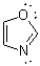
A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following best describes the cyclopentadienyl radical shown below? 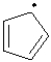
A)aromatic
B)anti-aromatic
C)non-aromatic
D)somewhat aromatic

A)aromatic
B)anti-aromatic
C)non-aromatic
D)somewhat aromatic

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
40
The bond length of a conjugated double bond is the same length as a non-conjugated double bond.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
41
The nitrogen atom in amide bonds is sp3 hybridized.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
42
Assuming planarity,the following ion is considered aromatic. 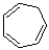


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
43
Lone pairs cannot be a part of conjugated systems.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
44
Alkynes cannot be a part of conjugated systems.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
45
Sigma bonds in conjugated systems have a larger energy barrier to rotation then sigma bonds in non-conjugated systems.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
46
The larger the difference in energy between the HOMO and LUMO,the larger the wavelength of light a molecule will absorb.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
47
Anti-aromatic compounds can be non-planar.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
48
Given the option,molecular structures tend to exist as non-aromatic instead of anti-aromatic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
49
Energy of light is inversely proportional to its wavelength.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
50
Any molecule that follows Hückel's rule is aromatic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
51
Assuming planarity,the following ion is considered anti-aromatic. 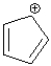


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
52
All lone pair electrons are considered ð electrons.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
53
All planar molecules are considered aromatic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
54
A molecule must be planar to be considered aromatic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
55
Carbanions are anti-aromatic,whereas carbocations are aromatic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
56
Assuming planarity,the following molecule is considered aromatic. 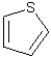


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
57
Amide bonds undergo free rotation at room temperature.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
58
Only ð electrons contribute towards aromaticity.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
59
Anti-aromatic compounds are also considered non-aromatic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
60
Assuming planarity,the following molecule is considered non-aromatic. 


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
61
Benzene has a _______________ heat of hydrogenation compared to the corresponding 1,3,5-hexatriene.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
62
A molecule that is anti-aromatic has _______________ electrons in its conjugated ring system.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
63
Aromatic double bonds tend to be _______________ stable than their corresponding non-aromatic double bonds.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
64
Both pyridine and pyrrole shown below have a lone pair of electrons on their nitrogen capable of acting as a base.Which compound would you expect to be the stronger base.Why? 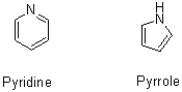


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
65
The hybridization of a nitrogen atom in an amide bond is _______________ hybridized.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
66
LUMO is an acronym for the _______________ .

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
67
_______________ circles are a method of mapping out aromatic orbital diagrams.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
68
Indicate which atoms are part of the conjugated system shown below 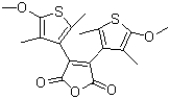


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
69
The following molecule shown below is Lipitor,a drug prescribed to lower cholesterol levels.How many aromatic rings does it contain,how many ð electron pairs are part of its conjugated system,and how many atoms are part of its conjugated system? 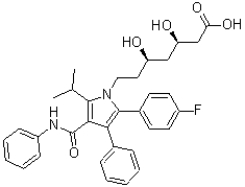


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
70
A molecule that is aromatic has _______________ electrons in its conjugated ring system.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
71
Draw two other resonance states of naphthalene showing electron flow.Predict whether you expect the bond between C1-C2 or C2-C3 to be longer in the figure shown below. 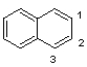


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
72
Benzyne (shown below)is a highly reactive yet still aromatic derivative of benzene.It is planar and cyclic,but has eight ð electrons instead of six.Explain how it is still aromatic yet but still defies Hückel's rule. 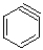


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
73
Demonstrate why in compound A,the nitrogen is more likely to act as a base,whereas in compound B,the oxygen atom is more likely to act as a base.Use resonance structures to justify your answers. 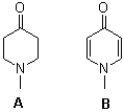


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
74
Conjugated systems tend to add rigidity to molecules.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
75
Which molecule shown below would you expect to have the larger dipole moment.Draw resonance structures using electron flow arrows for both molecules to show why. 


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
76
HOMO is an acronym for the _______________ .

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
77
Show electron flow and draw a resonance state to show why the following molecule is aromatic. 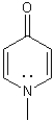


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
78
A molecule that follows all criteria for aromaticity except only contains 4n delocalized electrons in the ring is said to be _______________.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
79
Two molecular orbitals that lie in the same energy are said to be _______________ .

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
80
Indicate which bonds are rotatable in the heterocyclic molecule Kibdelone C shown below,ignoring methyl groups. 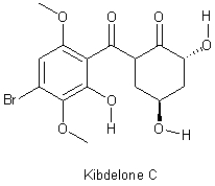


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck



