Deck 8: Ð Bonds As Nucleophiles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/96
Play
Full screen (f)
Deck 8: Ð Bonds As Nucleophiles
1
In the electrophilic addition of HBr to an alkene,what does the pi bond act as?
A)a nucleophile
B)an electrophile
C)a leaving group
D)a Lewis acida
A)a nucleophile
B)an electrophile
C)a leaving group
D)a Lewis acida
a nucleophile
2
What is the driving force for the brominium ion formation in the addition of Br2 to an alkene? 
A)Bromine is more electronegative than carbon and can thus better handle the positive charge.
B)The positively charged bromine has a full octet,whereas the positively charged carbon does not.
C)The cyclic structure of the brominium ion introduces added stability over the straight chain form.
D)The carbocation structure is not capable of hyperconjugation,whereas the bromonium ion is capable of hyperconjugation.

A)Bromine is more electronegative than carbon and can thus better handle the positive charge.
B)The positively charged bromine has a full octet,whereas the positively charged carbon does not.
C)The cyclic structure of the brominium ion introduces added stability over the straight chain form.
D)The carbocation structure is not capable of hyperconjugation,whereas the bromonium ion is capable of hyperconjugation.
The positively charged bromine has a full octet,whereas the positively charged carbon does not.
3
Which of the following reaction conditions would achieve the transformation shown below? 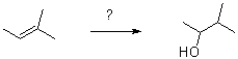
A)H2O,H2SO4
B)1)Hg(OAc)2,H2O 2)NaBH4
C)1)BH3 2)H2O2,NaOH,H2O
D)1)mCPBA 2)H+,H2O

A)H2O,H2SO4
B)1)Hg(OAc)2,H2O 2)NaBH4
C)1)BH3 2)H2O2,NaOH,H2O
D)1)mCPBA 2)H+,H2O
1)BH3 2)H2O2,NaOH,H2O
4
What is the major product of the following reaction? 
A)
B)
C)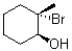
D)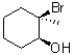
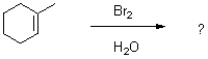
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
5
What sort of intermediate leads to the regiochemistry observed in the addition of hydrobromic acid to an alkene?
A)carbocation
B)bromonium ion
C)carbene
D)bromide
A)carbocation
B)bromonium ion
C)carbene
D)bromide

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
6
What sort of stereoselectivity is attained upon the addition of bromine to an alkene?
A)Markovnikov
B)anti-Markovnikov
C)syn
D)anti
A)Markovnikov
B)anti-Markovnikov
C)syn
D)anti

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
7
What is (are)the product(s)of the following electrophilic addition? 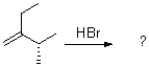
A)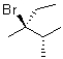
B)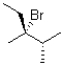
C)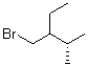
D)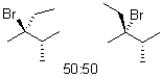

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
8
What would be the major product of the following reaction performed under kinetic conditions? 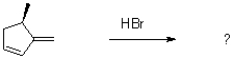
A)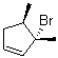
B)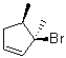
C)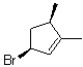
D)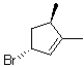

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
9
What is the hybridization of the positively charged carbon in the carbocation showed below? 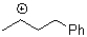
A)S
B)Sp
C)sp2
D)sp3

A)S
B)Sp
C)sp2
D)sp3

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
10
What sort of regiochemistry is attained upon the addition of water to an alkene by oxymercuration-demurcuration?
A)Markovnikov
B)anti-Markovnikov
C)syn
D)anti
A)Markovnikov
B)anti-Markovnikov
C)syn
D)anti

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
11
What is(are)the product(s)of the following electrophilic addition? 
A)
B)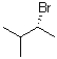
C)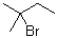
D)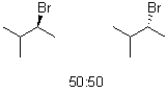
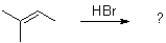
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the kinetic and thermodynamic products of the following reaction: 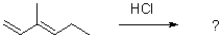
A)kinetic: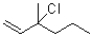 thermodynamic:
thermodynamic:
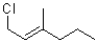
B)kinetic: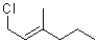 thermodynamic:
thermodynamic:
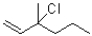
C)kinetic: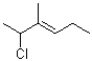 thermodynamic:
thermodynamic:
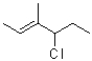
D)kinetic:
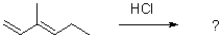
A)kinetic:
 thermodynamic:
thermodynamic:
B)kinetic:
 thermodynamic:
thermodynamic:
C)kinetic:
 thermodynamic:
thermodynamic:
D)kinetic:

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the two carbocations shown below.Which is more stable and why? 
A)I because of sterics
B)I because of hyperconjugation
C)II because of sterics
D)II because of hyperconjugation

A)I because of sterics
B)I because of hyperconjugation
C)II because of sterics
D)II because of hyperconjugation

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
14
Which product would be expected in the hydrogenation of 3-methylhept-3-ene with excess hydrogen gas using a palladium catalyst?
A)(R)-3-methylheptane
B)(S)-3-methylheptane
C)racemic 3-methylheptane
D)No reaction would occur.c
A)(R)-3-methylheptane
B)(S)-3-methylheptane
C)racemic 3-methylheptane
D)No reaction would occur.c

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following reaction conditions would achieve the transformation shown below? 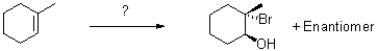
A)Br2,H2O
B)1)mCPBA 2)NaBr,NH4Cl,H2O
C)1)BH3,H2O2 2)Br2
D)NaBr,NH4Cl,H2O
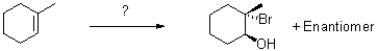
A)Br2,H2O
B)1)mCPBA 2)NaBr,NH4Cl,H2O
C)1)BH3,H2O2 2)Br2
D)NaBr,NH4Cl,H2O

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
16
How many stereoisomers would be expected to form given the following halohydrin reaction? 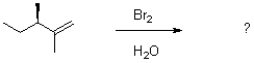
A)1
B)2
C)4
D)8
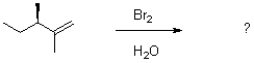
A)1
B)2
C)4
D)8

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
17
What type of bonds comprise a carbon-carbon double bond?
A)two sigma bonds
B)two pi bonds
C)one sigma and one pi bond
D)one pi bond
A)two sigma bonds
B)two pi bonds
C)one sigma and one pi bond
D)one pi bond

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following represents the most stable carbocation?
A)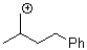
B)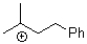
C)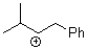
D)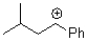
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
19
What sort of regiochemistry is attained upon the acid catalyzed addition of water to an alkene?
A)Markovnikov
B)anti-Markovnikov
C)syn
D)anti
A)Markovnikov
B)anti-Markovnikov
C)syn
D)anti

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
20
In the addition of bromine to an alkene,how does the bromonium ion behave?
A)as a Brønsted acid
B)as a Brønsted base
C)as a nucleophile
D)as an electrophile
A)as a Brønsted acid
B)as a Brønsted base
C)as a nucleophile
D)as an electrophile

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
21
How many stereoisomers would be expected to form given the following epoxide ring opening reaction? 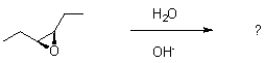
A)1
B)2
C)3
D)4
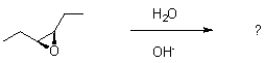
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following reaction conditions would achieve the transformation shown below? 
A)H2O,H2SO4
B)1)Hg(OAc)2,H2O 2)NaBH4
C)1)BH3 2)H2O2,NaOH,H2O
D)H2O,NaOH

A)H2O,H2SO4
B)1)Hg(OAc)2,H2O 2)NaBH4
C)1)BH3 2)H2O2,NaOH,H2O
D)H2O,NaOH

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
23
If deuterated borane were used instead of borane in the hydroboration oxidation of the alkene shown below,what would be the expected product? (D = 2H) 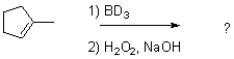
A)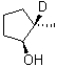
B)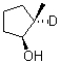
C)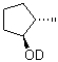
D)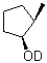
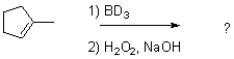
A)

B)

C)

D)
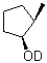

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
24
What would the major product of the following reaction be? 
A)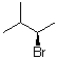
B)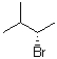
C)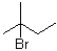
D)a racemic mixture of

A)

B)

C)

D)a racemic mixture of

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
25
What would be the expected product of the reaction shown below? 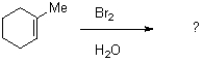
A)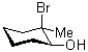
B)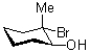
C)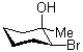
D)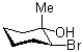
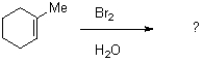
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
26
What would be the expected product(s)for the following transformation? 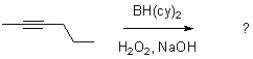
A)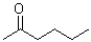
B)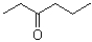
C)No reaction would occur.
D)A 50:50 mixture of
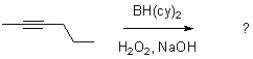
A)

B)

C)No reaction would occur.
D)A 50:50 mixture of

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following best describes the formation of an epoxide from cyclopentene with mCPBA?
A)stereoselective
B)stereospecific
C)regioselective
D)regiospecific
A)stereoselective
B)stereospecific
C)regioselective
D)regiospecific

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
28
In the following base catalyzed epoxide ring opening reaction,which stereoisomer do you expect to be the major product? 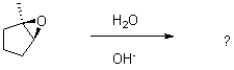
A)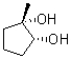
B)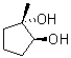
C)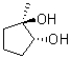
D)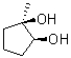

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following best represents the transition state leading to the first intermediate in the following hydroboration oxidation reaction? 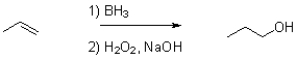
A)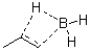
B)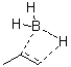
C)
D)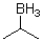

A)
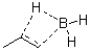
B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the two products shown below would be expected to be the major product and why? 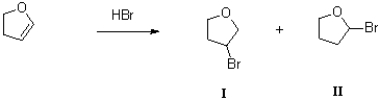
A)I because of resonance stabilization by the oxygen lone pairs
B)I because of induction from the oxygen atom
C)II because of induction from the oxygen atom
D)II because of resonance stabilization by the oxygen lone pairs

A)I because of resonance stabilization by the oxygen lone pairs
B)I because of induction from the oxygen atom
C)II because of induction from the oxygen atom
D)II because of resonance stabilization by the oxygen lone pairs

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following best describes a stereoselective reaction?
A)a reaction where one stereoisomer is produced more than another
B)a reaction that exclusively produced only one enantiomer
C)a reaction that forms a stereocentre
D)a reaction that involves a chiral reactant
A)a reaction where one stereoisomer is produced more than another
B)a reaction that exclusively produced only one enantiomer
C)a reaction that forms a stereocentre
D)a reaction that involves a chiral reactant

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following best describes the product of the hydroboration oxidation of an alkene?
A)syn addition and Markovnikov
B)anti addition and Markovnikov
C)syn addition and anti-Markovnikov
D)anti addition and anti-Markovnikov
A)syn addition and Markovnikov
B)anti addition and Markovnikov
C)syn addition and anti-Markovnikov
D)anti addition and anti-Markovnikov

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
33
What would be the expected product from the reaction shown below? 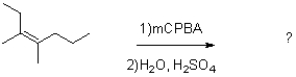
A)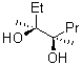
B)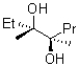
C)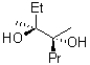
D)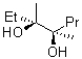
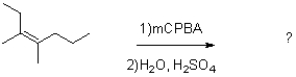
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
34
What would be the expected product for the epoxidation of but-2-ene (shown below)? 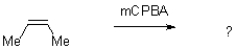
A)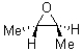
B)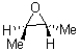
C)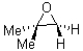
D)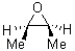
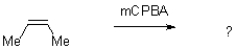
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements best defines the Hammond postulate?
A)The kinetic pathway is the pathway with the lowest energy transition state.
B)The rate of a reaction depends entirely on the activation energy barrier.
C)The structure of an intermediate most resembles the species nearest to it in energy.
D)The structure of a transition state most resembles the species nearest to it in energy.
A)The kinetic pathway is the pathway with the lowest energy transition state.
B)The rate of a reaction depends entirely on the activation energy barrier.
C)The structure of an intermediate most resembles the species nearest to it in energy.
D)The structure of a transition state most resembles the species nearest to it in energy.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
36
What sort of selectivity would be achieved from the reaction shown below? 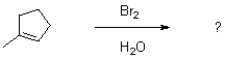
A)stereoselectivity
B)regioselectivity
C)stereoselectivity and regioselectivity
D)no selectivity
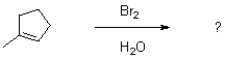
A)stereoselectivity
B)regioselectivity
C)stereoselectivity and regioselectivity
D)no selectivity

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following electrophilic addition reactions is both regioselective and stereoselective?
A)oxymercuration-demercuration
B)hydroboration oxidation
C)hydrogen halide addition
D)halogen addition
A)oxymercuration-demercuration
B)hydroboration oxidation
C)hydrogen halide addition
D)halogen addition

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
38
What would be expected to be the major product under the reaction conditions shown below? 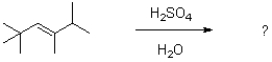
A)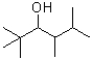
B)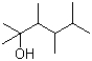
C)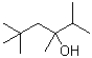
D)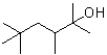
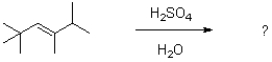
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
39
Why are reactions involving a carbocation intermediate considered NOT stereoselective?
A)Carbocations can undergo carbocation rearrangements,leading to a mixture of stereoisomers.
B)Carbocations are highly reactive,resulting in a loss of stereoselectivity.
C)The newly formed empty p orbital is accessible above and below the plane of the molecule.
D)All carbocations are achiral molecules.
A)Carbocations can undergo carbocation rearrangements,leading to a mixture of stereoisomers.
B)Carbocations are highly reactive,resulting in a loss of stereoselectivity.
C)The newly formed empty p orbital is accessible above and below the plane of the molecule.
D)All carbocations are achiral molecules.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
40
What would be the expected product from the reaction shown below? 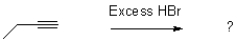
A)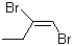
B)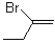
C)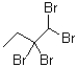
D)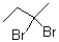
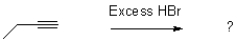
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
41
What kind of product would result from the reduction of an internal alkyne using hydrogen gas and Lindlar's catalyst?
A)a product with trans- stereochemistry
B)a product with cis- stereochemistry
C)a product with anti- stereochemistry
D)no stereochemistry
A)a product with trans- stereochemistry
B)a product with cis- stereochemistry
C)a product with anti- stereochemistry
D)no stereochemistry

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
42
Why is anti-Markovnikov regiochemistry primarily observed in hydroboration oxidation of alkenes?
A)It is a result of the kinetic pathway.
B)It is a result of the thermodynamic pathway.
C)The reaction uses boron's empty p-orbitals instead of an empty carbocation p-orbital.
D)The first step in the mechanism is concerted.
A)It is a result of the kinetic pathway.
B)It is a result of the thermodynamic pathway.
C)The reaction uses boron's empty p-orbitals instead of an empty carbocation p-orbital.
D)The first step in the mechanism is concerted.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following best describes the formation of an alcohol from an alkene using either oxomercuration or acid-catalyzed hydrolysis?
A)Both result in a Markovnikov product.
B)Both are susceptible to carbocation rearrangements.
C)Both are stereoselective.
D)Neither are regioselective.
A)Both result in a Markovnikov product.
B)Both are susceptible to carbocation rearrangements.
C)Both are stereoselective.
D)Neither are regioselective.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
44
A reaction that produces a chiral product is considered stereoselective.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
45
Addition of bromine to an alkene leads to a Markovnikov product.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
46
All electrophilic addition reactions to alkenes are regioselective.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
47
What would be the kinetic product of one equivalent of water reacting with 4-methylhex-1,4-diene under acidic conditions?
A)4-methylhex-1,4-diol
B)4-methylhex-1-en-4-ol
C)4-methylhex-4-en-1-ol
D)3-methylhex-5-en-3-ol
A)4-methylhex-1,4-diol
B)4-methylhex-1-en-4-ol
C)4-methylhex-4-en-1-ol
D)3-methylhex-5-en-3-ol

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
48
Markovnikov regiochemistry is a result of both carbocation stability and sterics.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
49
A secondary carbocation neighbouring a tertiary carbon is at risk for carbocation rearrangements.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
50
Hydrogenation of an alkene using palladium as a catalyst results in syn addition.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
51
The reaction of a terminal alkyne with Hg(AcO)2 results in an aldehyde.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
52
Hydroboration-oxidation of alkenes results in anti-Markovnikov stereochemistry.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
53
What would be the expected product of one equivalent of bromine with 3-methybutyne?
A)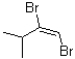
B)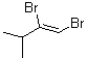
C)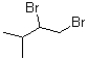
D)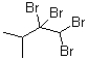
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is observed in the hydrogenation of an alkene in the presence of a palladium catalyst?
A)Markovnikov addition
B)anti-Markovnikov addition
C)syn addition
D)anti addition
A)Markovnikov addition
B)anti-Markovnikov addition
C)syn addition
D)anti addition

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
55
In electrophilic addition reactions to alkenes,the ð electrons act as an electrophile.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following would be an expected intermediate under the reaction conditions shown below? 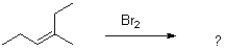
A)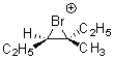
B)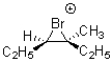
C)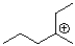
D)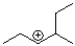
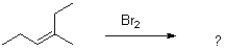
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
57
What would be the expected product or products of (R)-3-chlorobut-1-ene with HBr?
A)(2R,3R)2-bromo-3-chlorobutane
B)(2S,3R)2-bromo-3-chlorobutane
C)a diastereomeric mixture of (2R,3R)2-bromo-3-chlorobutane and (2S,3R)2-bromo-3-chlorobutane
D)meso 2-bromo-3-chlorobutane
A)(2R,3R)2-bromo-3-chlorobutane
B)(2S,3R)2-bromo-3-chlorobutane
C)a diastereomeric mixture of (2R,3R)2-bromo-3-chlorobutane and (2S,3R)2-bromo-3-chlorobutane
D)meso 2-bromo-3-chlorobutane

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following alkenes would react the quickest in the presence of sulfuric acid and water?
A)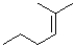
B)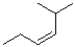
C)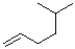
D)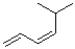
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
59
Hydroboration-oxidation of an alkene results in syn addition.
8
8

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
60
How many carbons are part of the conjugated system in the molecule shown below? 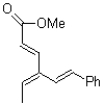
A)6
B)7
C)13
D)15

A)6
B)7
C)13
D)15

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
61
The reaction of an internal alkyne with BH(cy)2 results in an aldehyde.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
62
Hydrogenation of an alkyne with Lindlar's catalyst results in a saturated product.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
63
Oxymercuration-demercuration of alkenes are at risk of carbocation rearrangements.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
64
Conjugation leads to stabilization of carbocations through induction.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
65
Carbocations formed in electrophilic addition reactions are_______________ hybridized.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
66
Alkynes can be converted to alcohols with two equivalents of water.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
67
Reactions involving a carbocation intermediate are not stereoselective.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
68
Acid catalyzed hydration of (S)-4-methylpent-1-ene results in the formation of alcohols that are enantiomers of each other.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
69
Hydrolysis of a bromonium leads to a product with anti-stereoselectivity.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
70
Reactions going through the more stable carbocation result in _______________ regiochemistry.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
71
The first step in the mechanism of bromine addition to an alkene is a(n)_______________ step.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
72
Conjugated alkenes form stable carbocations in addition reactions,leading to slower reaction rates.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
73
Epoxides can be hydrolyzed in the presence of both base and acid.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
74
Hydrogenation of an alkyne with Lindlar's catalyst results in a product with cis stereochemistry.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
75
Hydrogen bromide addition to an alkyne goes through a carbocation intermediate.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
76
The product that forms fastest is considered the _______________ product.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
77
Reacting an alkyne with only one equivalent of hydrogen gas in the presence of a palladium catalyst results in an alkene as a primary product.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
78
Addition of atoms to the same face of an alkene is an example of regioselectivity.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
79
A carbocation is an example of a Lewis acid.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
80
Acid hydrolysis of an epoxide results in anti-addition.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck



