Deck 5: Organic Reaction Mechanisms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 5: Organic Reaction Mechanisms
1
Which of the following has the biggest contribution to a significant resonance form?
A)the most atoms with full octets
B)the fewest number of formal charges
C)the largest spread of like formal charges
D)the negative formal charges on the most electronegative atoms
A)the most atoms with full octets
B)the fewest number of formal charges
C)the largest spread of like formal charges
D)the negative formal charges on the most electronegative atoms
the most atoms with full octets
2
What do curved arrows in organic reaction mechanisms represent?
A)the flow of electrons in a reaction
B)the flow of atoms in a reaction
C)the breaking of bonds in a reaction
D)the flow of positive charge in a reaction
A)the flow of electrons in a reaction
B)the flow of atoms in a reaction
C)the breaking of bonds in a reaction
D)the flow of positive charge in a reaction
the flow of electrons in a reaction
3
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,what is the formal charge of the oxygen atom at B?
A)+1
B)0
C)-1
D)-2
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,what is the formal charge of the oxygen atom at B?
A)+1
B)0
C)-1
D)-2
-1
4
What is the formal charge of the nitrogen atom in the structure shown below? 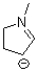
A)-1
B)0
C)+1
D)+2

A)-1
B)0
C)+1
D)+2

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following best describes carbanions?
A)They are Lewis acids and electrophilic.
B)They are Lewis bases and electrophilic.
C)They are Lewis acids and nucleophilic.
D)They are Lewis bases and nucleophilic.
A)They are Lewis acids and electrophilic.
B)They are Lewis bases and electrophilic.
C)They are Lewis acids and nucleophilic.
D)They are Lewis bases and nucleophilic.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following best describes an intramolecular reaction?
A)Only a single molecule is involved.
B)Only a single functional group is involved.
C)More than one molecule is involved.
D)More than one curved arrow is involved.
A)Only a single molecule is involved.
B)Only a single functional group is involved.
C)More than one molecule is involved.
D)More than one curved arrow is involved.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,what is the formal charge of the nitrogen atom at A?
A)+1
B)0
C)-1
D)-2
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,what is the formal charge of the nitrogen atom at A?
A)+1
B)0
C)-1
D)-2

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
how many lone pair electrons are around the negatively charged carbon atom shown below? 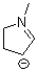
A)0
B)1
C)2
D)3

A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following arrows represents resonance between two structures?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is NOT a resonance structure of the amide bond shown below? 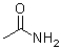
A)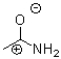
B)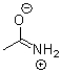
C)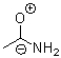
D)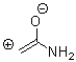

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
What is the formal charge around the oxygen atom shown below? 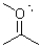
A)-2
B)-1
C)0
D)+1

A)-2
B)-1
C)0
D)+1

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
what is the formal charge of each of the nitrogen atoms in the azide functional group shown below. 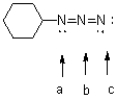
A)a = 0,b = +1,c = 0
B)a = +1,b = -1,c = 0
C)a = 0,b = +1,c = -1
D)a = -1,b = +1,c = -1

A)a = 0,b = +1,c = 0
B)a = +1,b = -1,c = 0
C)a = 0,b = +1,c = -1
D)a = -1,b = +1,c = -1

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,how many lone pairs of electrons can be found on the nitrogen atom at A?
A)0
B)1
C)2
D)3
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,how many lone pairs of electrons can be found on the nitrogen atom at A?
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,which of the following best describes the resonance states I and II?
A)I is more stable than II because the formal negative charge is on a more electronegative atom.
B)I is more stable than II because all the atoms follow the octet rule.
C)II is more stable than I because the formal negative charge is on a more electronegative atom.
D)II is more stable than I because all the atoms follow the octet rule.
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,which of the following best describes the resonance states I and II?
A)I is more stable than II because the formal negative charge is on a more electronegative atom.
B)I is more stable than II because all the atoms follow the octet rule.
C)II is more stable than I because the formal negative charge is on a more electronegative atom.
D)II is more stable than I because all the atoms follow the octet rule.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following best describes an atom with an unpaired electron?
A)anion
B)cation
C)radical
D)nucleophile
A)anion
B)cation
C)radical
D)nucleophile

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,what is the formal charge of the nitrogen atom at C?
A)+1
B)0
C)-1
D)-2
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,what is the formal charge of the nitrogen atom at C?
A)+1
B)0
C)-1
D)-2

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following best describes carbocations?
A)They are electrophilic and electron deficient.
B)They are electrophilic and electron rich.
C)They are nucleophilic and electron deficient.
D)They are nucleophilic and electron rich.
A)They are electrophilic and electron deficient.
B)They are electrophilic and electron rich.
C)They are nucleophilic and electron deficient.
D)They are nucleophilic and electron rich.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
What is the formal charge of the nitrogen atom shown below? 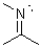
A)-3
B)0
C)+1
D)+5

A)-3
B)0
C)+1
D)+5

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following best describes the resonance forms of an amide bond shown below? 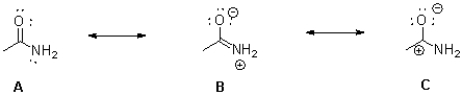
A)A is the largest contributor;B is the smallest contributor.
B)A is the largest contributor;C is the smallest contributor.
C)B is the largest contributor;C is the smallest contributor.
D)C is the largest contributor;B is the smallest contributor.

A)A is the largest contributor;B is the smallest contributor.
B)A is the largest contributor;C is the smallest contributor.
C)B is the largest contributor;C is the smallest contributor.
D)C is the largest contributor;B is the smallest contributor.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following represents the correct arrow flow in the reaction shown below? 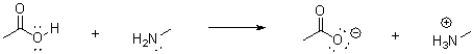
A)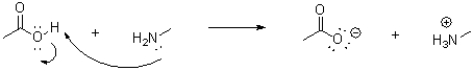
B)
C)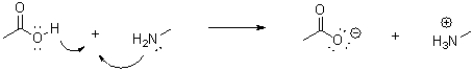
D)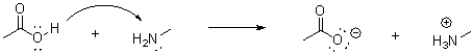

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
Curved arrows in reaction mechanism go from a positive site to a negative site.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
Intramolecular reactions involve only a single reactant.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following compounds is NOT a resonance form of the species shown below? 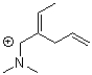
A)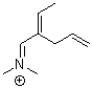
B)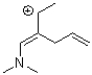
C)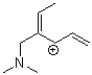
D)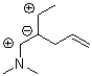

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,how many lone pairs of electrons can be found on the nitrogen atom at F?
A)0
B)1
C)2
D)3
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,how many lone pairs of electrons can be found on the nitrogen atom at F?
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
What is the product or products of the following arrow pushing? 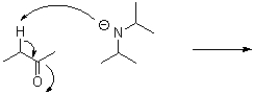
A)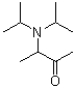
B)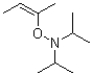
C)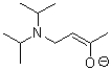
D)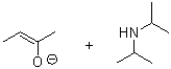

A)

B)

C)

D)
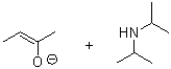

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
Given the Lewis structure shown below,what are the formal charges of the sulfur and oxygen atoms? 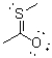
A)sulfur = +1,oxygen = -1
B)sulfur = 0,oxygen = 0
C)sulfur = -1 oxygen = +1
D)sulfur = 0 oxygen = -1

A)sulfur = +1,oxygen = -1
B)sulfur = 0,oxygen = 0
C)sulfur = -1 oxygen = +1
D)sulfur = 0 oxygen = -1

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
Both ð and ó bonds can be involved in resonance.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,how many lone pairs of electrons can be found on the nitrogen atom at E?
A)0
B)1
C)2
D)3
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,how many lone pairs of electrons can be found on the nitrogen atom at E?
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
What is the product or products of the following arrow pushing? 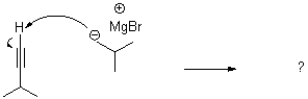
A)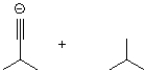
B)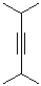
C)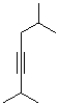
D)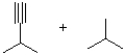

A)
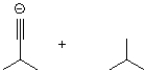
B)
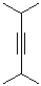
C)

D)
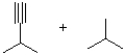

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
Curved arrows in reaction mechanism show the flow of atoms.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,how many lone pairs of electrons can be found on the oxygen atom at B?
A)0
B)1
C)2
D)3
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,how many lone pairs of electrons can be found on the oxygen atom at B?
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
Given the following Lewis structure,how many lone pairs are expected to be around the sulfur and oxygen atom? 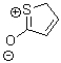
A)sulfur = 2,oxygen = 2
B)sulfur = 1,oxygen = 2
C)sulfur = 2 oxygen = 3
D)sulfur = 1 oxygen = 3

A)sulfur = 2,oxygen = 2
B)sulfur = 1,oxygen = 2
C)sulfur = 2 oxygen = 3
D)sulfur = 1 oxygen = 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,how many lone pairs of electrons can be found on the nitrogen atom at C?
A)0
B)1
C)2
D)3
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,how many lone pairs of electrons can be found on the nitrogen atom at C?
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,how many lone pairs of electrons can be found on the carbon atom at D?
A)0
B)1
C)2
D)3
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,how many lone pairs of electrons can be found on the carbon atom at D?
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the most important guideline when assessing the quality of resonance forms?
A)the fewest number of formal charges
B)negative charges located on the most electronegative atoms
C)positive charges located on the least electronegative atoms
D)the most atoms with the full octets
A)the fewest number of formal charges
B)negative charges located on the most electronegative atoms
C)positive charges located on the least electronegative atoms
D)the most atoms with the full octets

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,what is the formal charge of the nitrogen atom at E?
A)-1
B)0
C)+1
D)+2
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,what is the formal charge of the nitrogen atom at E?
A)-1
B)0
C)+1
D)+2

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,what is the formal charge of the carbon atom at D?
A)+1
B)0
C)-1
D)-2
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,what is the formal charge of the carbon atom at D?
A)+1
B)0
C)-1
D)-2

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.
Referring to Figure 1,what is the formal charge of the nitrogen atom at F?
A)-1
B)0
C)+1
D)+2
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted.

Referring to Figure 1,what is the formal charge of the nitrogen atom at F?
A)-1
B)0
C)+1
D)+2

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
Different curved arrow shapes exist for one and two electron movement.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
Curved mechanism arrows flow from nucleophile to electrophile.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
Curved arrows always represent the flow of electron pairs.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
Curved arrows in reaction mechanism show both bond breaking and bond forming.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
Atoms with full octets have more of an influence on the contribution of a resonance form than the amount of formal charges.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
Resonance structures are in equilibrium with each other.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
Resonance structures require at least two neighboring atoms with p-orbitals.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
A carbocation contains a p orbital capable of receiving electrons to form a new bond.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
A resonance form is a blend of all resonance forms at the same time.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
All resonance forms contribute equally to a resonance hybrid.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
Structures with unpaired electrons are also referred to as radicals.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
When evaluating resonance forms,an optimal structure is one that places positive formal charges on atoms with high electronegativity.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
An oxygen atom with two ó bonds,one ð bond,and a lone pair of electrons has a formal charge of -1.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
All atoms with positive charge can receive electrons through bond formation.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
Delocalization of electrons occurs over p orbitals that are parallel AND perpendicular to each other.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
Resonance has no effect on the stability of a structure.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
A ð bond between two atoms with different electronegativities can undergo resonance.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
Atoms with arrows pointing away from them increase in negative charge.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
Structures in resonance exist with distinct chemical structures.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
A structure that contains an atom with an unpaired electron is called a(n)_______________ ,

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
Atoms with full octets have no influence on the contribution of individual resonance forms.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
All resonance forms have equal contribution to a resonance hybrid.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
The formal charge of a sulfur atom with one ó bond,one ð bond,and two lone pairs of electrons is _______________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
A reaction with curved arrows moving between two different molecules is an example of a(n)_______________ reaction.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following compounds would you expect to have greater resonance stabilization.Use curved arrows and resonance structures to demonstrate why. 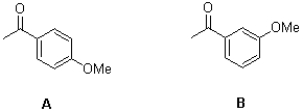


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
Insert in all formal charges of the product from the arrow pushing shown below.Add all lone pairs to the product. 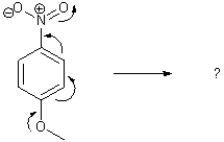


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
Draw all the resonance structures of the p-dimethylamino pyridinium ion shown below.Use curved arrows to transition from one resonance form to another. 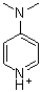


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
Draw the product(s)of the following arrow pushing shown below.Include all formal charges. 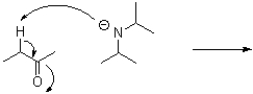


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
A curved arrow pointing to an atom in a reaction mechanism _______________ its charge integer.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
Insert curved arrows in 7-dehydrocholesterol to show how it becomes Previtamin D. 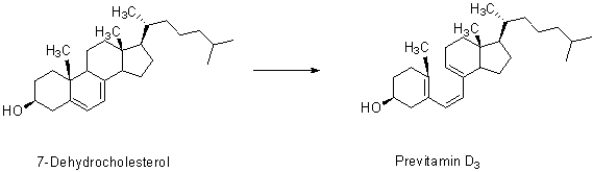


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
The formal charge of a Boron atom with four ó bonds is _______________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
Draw curved arrows to show electron flow in the following transformation. 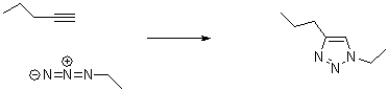


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
Different resonance forms of a structure are separated by a(n)_______________ arrow

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
The movement of a single electron in a reaction mechanism involves the use of a(n)_______________ arrow

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
Using curved arrows,show the flow of electrons to obtain the greatest resonance contributor to the following compound,and state why this is the greatest resonance contributor. 


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
A highly contributing resonance structure places positive formal charges on the _______________ electronegative atom.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
Use curved arrows to show the electron flow in the following transformation. 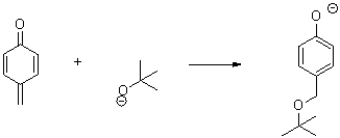


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
Curved arrows show the movement of _______________ in reaction mechanisms.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
Curved arrows within the same structure are an example of a(n)_______________ reaction.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck



